Hydrogen Bonding and Common Mistakes
HTML-код
- Опубликовано: 16 июн 2012
- To see all my Chemistry videos, check out
socratic.org/chemistry
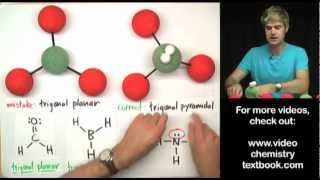
Hydrogen bonding can be so confusing, and in this video we talk about some common mistakes. Hydrogen bonds are intermolecular forces between molecules. They form because one atom has a high electronegativity, so it gets a partial negative charge, and the hydrogen gets a partial positive charge.








you put my college professors to shame. These people have PHDs and cannot convey this knowledge the way you do. That being said, the world could use a lot more people like you and a lot less professors like mine.
Analogies are always imprecise. But through my years in the classroom, I've never encountered any student confusion about this issue. Because, honestly, when students are learning this for the very first time, they just need an "image" (like magnets) to make the concept more relatable. It's only people like you and me (who understand the nuances between magnetic and electrostatic forces) who worry there'd be confusion. Most 14 year-olds just think "OK, they stick together like magnets."
Anyone from 2020 ?
Anyone here from 2021 .
2022 were you at
I am starting to think I do like chemistry
It''s funny because my chem teacher is terrible so this guy has been teaching me all semester xD
H is out here getting used and taken advantage of, she needs a real man that treats her right.
god has a special place in heaven for you
Bruh Owen Wilson and Justin Bieber’s child is a genius
even after 8 years this guy best teacher! Anyone here in 2020?
Hey everyone, I'm here to help. If you have any questions or just want to learn more, click on the link in the description above. It'll take you to a page where you can ask me questions.
OUT-f@cking-STANDING explanation. I should just pay my tuition dollars to you.
I spent the past couple of weeks not understanding intermolecular forces/hydrogen bonding at all!! My textbook was way too complicated to understand. I can't thank you enough for making this video, I finally understand this and I am so happy!! :)
best explanations one can find ... I am sticking to this channel for my bio class... thanks for sharing.
Thank you Sir that was a flawless explanation. THAT'S what I call a great video.
YOU'RE SO AMAZING, I MISSED THIS LESSON TODAY, U SAVED ME THAAAAAAAAAAAAAAANX ALOT
Coming from another teacher, you are a natural educator!! Very few people who are good at chemistry and mathematics can actually explain it so clearly, simply and thoroughly, so that it makes sense and can be visualized. It makes me sad that it is such a rare gift, had I been taught better in high school, I would've had a very different life and career. Anyway, well done and thank you so much for taking the time to do this, outstanding!
you really put a lot of effort on this video, and i also appreciate your creativity! support and hope you can work more on chemistry! thank you!
Loud and clear, and very easy to understand. Awesome, thank you.