The pH scale | Acids and bases | AP Chemistry | Khan Academy
HTML-код
- Опубликовано: 29 июл 2021
- Keep going! Check out the next lesson and practice what you’re learning:
www.khanacademy.org/science/a...
The pH scale is a convenient way to represent the acidity or basicity of a solution. We can calculate the pH of a solution by taking the negative logarithm of the hydronium ion concentration, or pH = -log[H₃O⁺]. At 25°C, a solution with a pH less than 7 is acidic, a solution with a pH greater than 7 is basic, and a solution with a pH equal to 7 is neutral. View more lessons or practice this subject at www.khanacademy.org/science/a...
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Donate or volunteer today! Donate here: www.khanacademy.org/donate?ut...
Volunteer here: www.khanacademy.org/contribut...
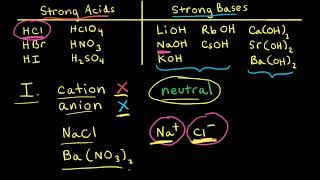








the explanation of how to ball park a pH without a calculator is gold
thank you!
Great video!
Brilliant
77°F
Thanks.
1
In conclusion, I find that the results of the equation in theory is based upon the negative reaction to the scale of relativity.
NEEEEEEERDDDDD