Brønsted-Lowry acids and bases | Chemical reactions | AP Chemistry | Khan Academy
HTML-код
- Опубликовано: 2 июл 2015
- In the Brønsted-Lowry definition of acids and bases, an acid is a proton (H⁺) donor, and a base is a proton acceptor. When a Brønsted-Lowry acid loses a proton, a conjugate base is formed. Similarly, when a Brønsted-Lowry base gains a proton, a conjugate acid is formed. A Brønsted-Lowry acid (or base) and its conjugate base (or acid) are known as a conjugate acid-base pair. View more lessons or practice this subject at www.khanacademy.org/science/a...
Khan Academy is a nonprofit organization with the mission of providing a free, world-class education for anyone, anywhere. We offer quizzes, questions, instructional videos, and articles on a range of academic subjects, including math, biology, chemistry, physics, history, economics, finance, grammar, preschool learning, and more. We provide teachers with tools and data so they can help their students develop the skills, habits, and mindsets for success in school and beyond. Khan Academy has been translated into dozens of languages, and 15 million people around the globe learn on Khan Academy every month. As a 501(c)(3) nonprofit organization, we would love your help!
Donate or volunteer today! Donate here: www.khanacademy.org/donate?ut...
Volunteer here: www.khanacademy.org/contribut...
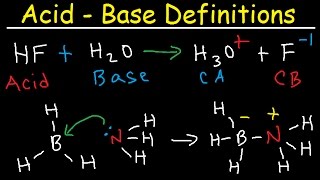







the best way to remember this is DAAB! donor-acid and accepter-base.
good to see that it helped someone
This makes me so angry. 10/10
Broo😢😢😢
100th like
BAAD for Lewis acids and bases
You are one of the best teachers in World for me. And I want to say a hearty thanks to the Khan Academy team for working for us.
Sometimes I wonder if this guy has a script or he knows every thing in khan academy
SkarrowJr. He writes things down prior to video
The scripts are written by other teachers as well which he gives credit on the website
I wish i had a teacher or a tutor to explain like this istg they explain stuff to me as if they’re running of time💔💔
thank you so much to Khan Academy for clearing the doubts of the students...... and to teach us in the easiest and the best way so we could understand
So donor is acid. And acceptor is base. Understood :) I feel so confident for chemistry exams now. Thank u :)
Excellent explanation .I understood it really well👍👍
This was helpful.. thanks 😊
Superb expalantion sir 👌👌👨🏫👨🏫and also very useful to me for examination thank you sir👨🏫👨🏫
u r just next to awesome
thanks bruh
thank u for this
Why does it favor the right?
It's really understandable
wow my concept super clear!
Can you please make a video on different examples of bronsted lowly concept of acids and bases
Lowry**
What the hell is the difference beetwen Arrhenius's and Bronsted-Lowry's theory??
Well this theory doesnt just limit a base to something that gives OH- ions. It can be base without releasing hydroxide or an acid without having the typical HCl kind of acid. Like water can be an acid or base in this theory , depending on the other chemical. I am a little late tho Lol
Katarina Pavlić yffuhh
3:38 why is he saying "both of these electrons in this pair" when hydrogen can only have 1 if bonded with a chlorine atom?
There will be one electron from the hydrogen and one from the chlorine to form a shared 'pair' of electrons.
thank you
عاشت ايدككك
Thank u💕❤️
Superb
Thankyou sirji
8:36
Good video but the repeating of words is very frustrating to watch.
The concept of not having an electron and being left only with a proton is all enough to remember both the lewis acid and bronsted acid .thank you sir
great video thanks!!
I still don't understand why is in the HCL , the electrons are grabbed by the chlorine atom? is it because of the electronegativity?
yes
Yep!
Yes.. Because electronegativity is the tendency of an atom to draw electrons closer to it. Chlorine is more electronegative and therefore chlorine takes the bond pair.
yes
@Mir Faraz Ali ironically i'm an architect-to-be haha
sir, if you could please explain to me the reason behind the hydrogen's electron being given up to chlorine and how can the h20 obtain a proton and how it did bond to hydrogen. please answer my question. ps: your explanation was very clear and straightforward. thanks for not using complicated words.
Yes i also have this question in my mind.
Does the base have to be water or can it be an other molecule as well?
it can be also other molecule
This is really useful thank u 😘😘😘😘
Hey! Doesn't the hydronium ion have a co-ordinate bond with the H from HCl??
Amaxing teacher!!!!
Amazing word reapeater
He said, this could give you a BASE Line",. get it a pun wow Sal!
Sir, I think Cl in the product should have an odd number of electron.
well....HCl has an electrovalent bond....not co valent
Very Good Presentation, very Nice voice delivery
I do have a doubt, why should electron move to a higher electron concentrated place?
H3O+ is formed by coordinate bond
At 4:03 he says that the water forms a covalent bond with the H+ from HCl, isn't that a dipole dipole hydrogen bond? is it an intra molecular bond?
It's a co-ordinate bond (also called a dative covalent bond) is a covalent bond (a shared pair of electrons) in which both electrons come from the same atom
@@TheVompom yep
I think HCl should be a gaseous form here instead of aqueous.
Gaseous HCl will not work...
Its a good video but i can´t undertad english lenguage very good So, Can you make subtittes in spanish? because y I dont understand the last minute. Thank you. (excuse me for my bad use lenguage)
So, do you still need it now? lol.
ZaiD Malik ^^'
Is ur English better now?
I don't understand anymore. . shouldn't a strong acid reject any kind of bonding?
But why a stable compound accept a proton
There's a ''thing'' called ''electronegetivity''.
Ahil Aaryan "Great" , "thanks"
Sorry the what definitions?
chlorine would have 7 electrons of its own nd it shares 1 electron with hydrogen
Sal do you have chemistry videos?
You're watching one.
Pathetic
Yes
Fuck, I'm gonna fail my exam tomorrow. 😂
100 th comments
Try not using pink pen. that' s not visible
why does the chloride anion have 8 electrons when it should have 7? am i the only one the confused ?
it's trying to fulfill the octet
rule
Mad chloride ion means cl- means 7 electrons before and after accepting an e`acquired 8
The same thing I didn't understand
why did u pronounce aqueous like ah-kwee-us
I still don't understand 😂
I am still 13 years old and i understood that! 🤓😅😅
stop repeating the words man hahah
♤♤♤♤♤♤♤♤♤
i kno its really distracting lol
+Mic Mcd 😴😫
It’s annoyinggggg
@@ShaVaughn He does that because saying it twice helps you remember it better. He's trying to help everyone watching.
Nice to watch but hate to hear reapeating words
1.75x speed gang because this dude repeats so much lol
2x actually geeze
This guy needs to retire!!!
The way he keeps repeating himself is so infuriating, he must be trying to teach really simple people
He is focusing too much on his accent and none efforts are left for explanation
His aqueous game is so strong