Practice Problem: Initial Rates and Rate Laws
HTML-код
- Опубликовано: 3 окт 2024
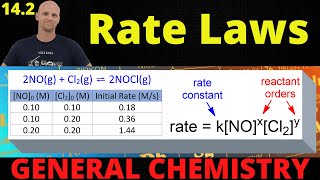
- To figure out the rate law for a reaction, we have to gather kinetic data. We can't know just by looking at the balanced equation. Let's practice using initial rates data to determine the rate law for a reaction!
Try all of the general chemistry practice problems: bit.ly/ProfDave...
General Chemistry Tutorials: bit.ly/ProfDave...
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: amzn.to/2HtNpVH
Bookshop: bit.ly/39cKADM
Barnes and Noble: bit.ly/3pUjmrn
Book Depository: bit.ly/3aOVDlT








Some days, I just want to give up and change my major. But then I remember Professor Dave's channel exists and that I'm not stupid, it is possible to learn the material. Thanks so much! :)
Savannah Adams
Nice! Which major are you?
@@ivoryas1696 I'm a biomedical sciences major at my uni :)
i can relate to this!
Professor Dave explains it in such a way that makes it easy to learn
Thank you, this was great. I did the problem on my own and then watched the remainder of the video.. I had almost everything correct except for the units in the answer for k!
Thanks Professor Dave! THIS HELPED!
Appreciate ur hardworking, thanks
thank you professor dave ,you are really awesome. I had this doubt for a long time and I got it clarified.
Just did this in CHM ll Thank you
Fun explanation by Professor Davs, or Professor William Williams...
I believe that there is an error in the final calculation of K. In the data we are given the rate of NO loss which is d[NO]/dt. However, The rate of reaction = -1.2 d[NO]/dt and when you calculated K you assumed that the Rate of NO loss (d[NO]/dt) is equal to the rate of the reaction which is not true. Can you please confirm?
thankyou
hello professor dave! can i use trial3/trial2 instead of trial3/trial1 when getting the x order?
Got a chem 1820 test...I'm rushing..if ykyk lol
But why we put concentration of trial 2 in numerator and of trial 1 in denomenator?
When i do it oppositely the answer is still the same I've got same order but is it necessary to put trial 2 concentrations in numerator??
How do I do one with 3 M Collins’s?
Won't the order for NO be 1 because as NO is multiplied by 1.5,rate is also multiplied by 1.5..but u wrote that it's multiplied by 2.25..i think there's an error here.
those are exponents
@@ProfessorDaveExplains yeah so if the rate increases in the same way as the concentration of NO. Wouldn't the exponent be 1 instead of 2?
sorry i misread at first, for NO the increase of rate is equal to the square of the increase of concentration, it's not by 1.5, it's by 2.25
@@ProfessorDaveExplains okay Thankss!
Sir , I can know evolution
I first
First