Practice Problem: Hess's Law
HTML-код
- Опубликовано: 16 окт 2024
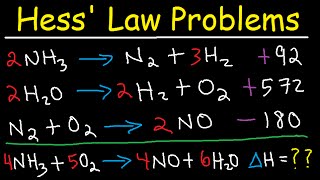
- If we want to find out some information regarding the enthalpy change of a reaction, but we don't want to perform the reaction, we can use Hess's law, which allows us to manipulate thermochemical data for related reactions. Let's practice this technique!
Try all of the general chemistry practice problems: bit.ly/ProfDave...
General Chemistry Tutorials: bit.ly/ProfDave...
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: amzn.to/2HtNpVH
Bookshop: bit.ly/39cKADM
Barnes and Noble: bit.ly/3pUjmrn
Book Depository: bit.ly/3aOVDlT








What's enthalpy? I don't understand ANY of this but I get a great sense of satisfaction knowing that someone out there does 👊😁👍
Enthalpy is the sum of internal energy and pressure volume work done. It is equivalent to the total heat supplied to the system.
@@saurabtharu7252 thanks bud
The total heat content of a body
Chemjesus, you are the only one who got the power to clear the concept within 4 min video! ✨ Thank you 😊🙏
It's been years since I've practiced any kind of engineering, and even longer since I've been in school, but this is still fun to listen to. (I'm also enjoying the Italian lessons) Thanks, Professor Dave! ❤️
Omg I have an exam on this in a couple of days, thank you! Perfect timing!
Have a test on energetics tomorrow and this popped up in my feed
Explanation was good👍👍👍
It's now clear to me
Hey Dave!! Love your channel. I am in ochem 2 and struggling with the Mannich reaction and was wondering if you were planning to post anything about it
i'll put it on the list!
So glad to see this video....Too much informative....your this short video has cleared my all concepts...
Yes. A quiz at 9. Perfect
Thanks sir
Needed this
Thanks Professor
For right video at right time😊😉
Thank you so much for your videos. I would like to get some references books names that you use for Chemistry..
Thanku so much sir🫡beautyy explanation❤
I did not understand this lesson at all, until I found your video.
Thank you so much for making this video Chemistry Jesus!!
This is Lloyd, the green ninja, signing off
this helped me so much your a life saverrr
excellent explanation!
Thanks great explanation!
Love you sir
what about when there are 2 compounds in one intermediate equation that both are unique to that one? Or also when none of the compounds in one intermediate equation are unique to that specific equation?
So much thanks Dave 🔥
Thank you so much sir
What if the given thermochemical equations have the same compound with what you're looking for, for example, for F2 then the given equations have F2, what equation to choose?
Yaay ! Did it allright..:)
Superb
God bless you
Professor dave #1
helpful
NYC sir
Thank you, Jesus.
You are teaching good but not sufficient for indian jee iit neet medical student try to improve.
Thank you sir