The WHOLE of Year 1 Inorganic Chemistry in 50 minutes - OCR A-Level
HTML-код
- Опубликовано: 26 июл 2024
- Recap Year 1/AS Chemistry!
This forms part of Paper 1 for OCR A-Level Chemistry.
You'll cover chapters 2-10 learning the key facts you need to pass your exams.
Please like, subscribe and share for daily revision videos.
Follow me on Instagram @999revisionemergency
Feel free to request topics or ask questions below.


![Finesse2Tymes - Pretty Ricky [Official Music Video]](http://i.ytimg.com/vi/xIoP_mhYRT0/mqdefault.jpg)




The video is very useful for few last days before the exam and congratulations on effort! One small mistake is at 30:00, the atomic radius increases down the group and decreases across the period.
Me at 9:50pm 👁👄👁
The test at 8:40am 😈
Hiya,
Firstly, amazing video thank you so much!!! Found it really useful
Secondly, I did something that might help.
Below is a time-topic list, so if you're looking for a specific topic you can skip.
Hope it helps.
0:00 - introduction
*2.1*
0:35 - Atoms and Subatomic Particles
1:11 - How Each Element Is Laid Out On The Periodic Table
1:24 - Isotopes
1:45 - Relative Masses And Formula To Calculate
2:19 - Elements, Compounds, Molecules And Ion Definitions
2:41 - The Periodic Table And Ions
3:30 - Polyatomic Ions To Memorise
3:50 - Ionic Compounds And Diatomic Molecules
4:32 - The Mole And Avagadro's Constant
5:27 - Empirical And Molecular Formula
6:06 - Water Of Crystallisation
6:38 - Mole Equations: Concentation And Volume
7:09 - The Ideal Gas Equation
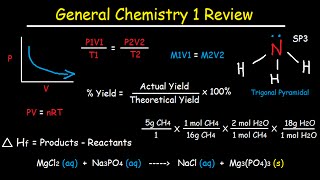
7:59 - Percentage Yield And Atom Economy
9:16 - Acids
9:45 - Bases, Alkalis And Neutralisation Equations
10:48 - Making A Standard Solution
11:44 - Titration And Percentage Uncertainty Formula
12:50 - REDOX
*2.2*
14:41 - Electron Configuration
18:43 - Ionic Bonding And Properties
19:52 - Covalent Bonding And Properties
20:54 - Shapes Of Molecules
22:48 - Electronegativity
25:05 - Dipole-Dipole Interactions
25:49 - Permanent-Permanent Dipole Interactions
26:32 - Hydrogen Bonding
*3.1*
27:40 - The Periodic Table Over Time
28:42 - Ionisation Energy
29:16 - Ionisation Energy Trends
30:20 - Exceptions For Ionisation Energy Trends
30:54 - Ionisation Energy Graph
31:13 - Metallic Bonding
32:57 - Group 2
34:42 - Group 7
36:05 - Group 7 Tests
36:28 - Disproportionation Reactions
36:56 - Ion Testing
*3.2*
38:15 - Enthalpy
38:33 - Standard Conditions
39:57 - Enthalpy Equation
40:52 - Enthalpy Definitions
41:36 - Average Bond Enthalpy/Bond Energies
42:07 - Hess' Law
43:22 - Rate Of Reaction
45:00 - Catalysts
45:57 - Boltzmann Distribution: Effects Of Temperature And Catalyst
47:23 - Equilibrium
47:41 - Le Chatelier's Principle And Factors Affecting Equilibirum's Position
48:59 - Equilibrium Constant
50:08 - End Of Lecture Message
*separated by module as according to contents page from textbook link below*
*www.worldofbooks.com/en-gb/books/victoria-stutt/ocr-as-a-level-chemistry-a-student-book-1-activebook/9781447990789?gclid=Cj0KCQjw7pKFBhDUARIsAFUoMDYIuvD5dVjbfSQO4UgOcjJZpuWC9NwOtLCoSrzhPqHDPUjoZu-zJVkaAsKhEALw_wcB*
THANK YOU SO MUCH!!
@@rmaissa1403 all good
pro trick : watch movies at Flixzone. I've been using it for watching a lot of movies recently.
@Tanner Jake Definitely, been watching on flixzone for months myself =)
Life saver
I've never been good with Chemistry, but this just helped me pass a difficult paper.
Thank you.
Keep up the great work.
Thank you so much! This helped me to consolidate all my knowledge of the Year 1 topics in one go and in a short amount of time. I'm was super stressed out because my end of year's start next week but this run through thorough all the topics has honestly really helped me. Thank youuuu :)
The units for pressure in the ideal gas equation is Pa not kPA!
can u make this for the year 2 content too ? it’ll be rlly useful for students having summer exams in 2022
thanks for all the support & ur hard work ❤️
Thank you for making this video, can you please create one on the whole of year 2 and organic chemistry please :)
This was so fun watching, thank you so much!
ruclips.net/channel/UCbrmz8Al45ZfKcRVS5eM7-w
2 x speed gang ✌
Damn, a whole year in _twenty-five_ minutes
It's the only way🙌
whole vid in 25 min
Great video 👍 lots of fun to watch 👍
hey, at 7:34 you should measure pressure in pascals not kilopascals. amazing video though!!
LIFE SAVERRRRRRRR!!!!!! :)
please please please do one for organic too?
yes, this would be pretty cool.
thank you for the video!!! one question , the energy of the 4s energy level is not less than the energy of 3p it is more... this is how it goes 1s,2s,2p,3s,sp,4s,3d
u......are........AMAZING!!!
can u pls do one of year 2 as well?
I procrastinate for 2 weeks, then revise half the entire course in 30 mins(x2 speed) 💀
27:00 "Ice is denser than liquid water". I don't think so, Sister.
ice IS denser
@@pogu2224 why does it float on water then? Ice has the highest density at around 4 °C in liquid state.
Ice is less dence than liquid h2o
Tp be honset This helps alot Thanks alot...upload more like this please...
Thank you so much for making this video
ice is not denser than water
Pressure is in pa not kpa:)
Thank You🙏
Thank you 😊
no bc ur acc a lifesaver tysmsmsmssmm
A small mistake at 7:40 , the unit of pressure is Pa not kPa
@@moban5660 attention to detail IS important so you should check ur facts, it’s measured in Pascals NOT kPa
@@moban5660 No,it’s measured in pascals,stop spreading false information and actually do some revision before spouting dumb comments
Didnt get full marks on pv=nrt cos of thid video
@@Louis-hi1xg Yh there are quite a few mistakes dotted around in the video
@@Louis-hi1xg 😂😂😂😂sorry to hear that man
Correct me if I’m wrong, but I thought at 16:25 The Subshell Order of Filling is meant to be: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p...?
No. You’re right!! Because it fills in order of increasing energy
@Tanush ! That is not the issue at all. When writing the electron config, you can write it either way around, and yes, it is preferred to write the 3d before the 4s.
Surprisingly, the issue isn't that she said 3d before 4s.
No, the issue is somehow worse, and very clear if you just click the timestamp in the comment - she specifically states that "the order of filling therefore is 1s, 2s, 2p, 3s, 4s, 3p, 4p, 4d." She doesn't even say 3d.
I've compiled a playlist of chemistry revision and haven't watched this video yet, but going by the comments and the sheer amount of errors, I think I'll be removing this one from the playlist. If only we had a way to show others with a simple counter that a video is bad, like a dislike button, _RUclips_
@@user-rv9vk8by5i exactly, she says that the 3p has a higher energy than the 4s 😭lmao
Doesn’t the atomic radius INCREASE down a group? Because more electron shells are being added 29:50
Yes
Yeah I think she got that wrong I searched it up aswell to make sure
@@omas1154 he*
Supposed to be 2 bonding and 2 lone pairs for bent or non linear
Do these notes align with the specification? super helpful btw, thank you! :)
how can we calculate how much energy in unit of electrovolt [eV] each level (shell) and each sublevel (s, p, d, f) has?
i don't take chemistry but i am now confident i will pass
33:18 if the Ca is reduced, how is it a reducing agent itself? Shouldn't it be an oxidising agent? Please clarify
can i use what i learned from this video for Organic Chemistry ?
26:56 ice is less dense than liquid water*
Thank you.
Can you upload link dor the slides please
18:55 How does Ionic Bonding form COVALENT lattices??
😂
dichromate ion is cr2o7^2- ! not cro7^2-
Dichromate 🥰
Interesting that this is called "Inorganic Chemistry"
In the US we refer to this as General Chemistry, or Gen Chem for short
Do you have a vid on Year 1 Organic Chem?
Can you solve this question?
Who is creat the word chemistry
At 3:44, dichromate is missing the "di" in the table. Thank you so much for this video
Huh?
“Metals always have a positive charge” Gold with the electronegativity of carbon: am I a joke to you?
here the night before the exam. I really hope this works lol
did it go well?
easy stuff
This is more like gen chem than college level inorganic for those looking to not waste their time…
Amazed you saw OCR A-Level Year 1, a whole english exam board with a spec called inorganic chem, and thought ah yea sure mate thisll be perfect for my college course. Fam this is for us stupid fuckers doing the eponymous OCR A-Level 🤧
Is this OCR A or OCR B?
A
couple of mistakes in this video but otherwise really helpful
🗿
Collision theory
collision theory is basically:
A reaction will not take place between two particles unless:
- they collide in the right direction and orientation
- they collide with an energy greater than the activation energy