Crystal Field Theory
HTML-код
- Опубликовано: 21 июл 2024
- This chemistry video tutorial provides a basic introduction into crystal field theory. It explains how to draw the crystal field splitting diagram of transition metal complex ions using weak field ligands and strong field ligands. It explains the difference between high spin and low spin diagrams and how to determine the magnetic properties of complex ions.
Get The Full 1 Hour Video:
/ mathsciencetutor
Direct Link to The Full Video:
bit.ly/3vShovS
Chemistry PDF Worksheets:
www.video-tutor.net/chemistry...
________________________
Full 1 Hour Video:
• Crystal Field Theory -...
RUclips Membership:
/ @theorganicchemistrytutor
Here is a list of topics in the full version of this video:
1. How To Draw The Crystal Field Splitting Diagram
2. Weak Field vs Strong Field Diagrams
3. Crystal Field Splitting Energy
4. How To Determine The Oxidation State of the Transition Metal In a Complex Ion
5. Understanding The Relative Splitting Energy of the 3D Orbital System In a Transition Metal
6. Crystal Field Theory - Basic Concepts
7. Ligands as Negative Point Charges
8. How To Determine The D Count of a Transition Metal Complex Ion
9. How To Write The Electron Configuration Using The T2g Set and Eg set
10. How To Calculate The Number of Unpaired Electrons In a Complex Ion
11. How To Determine The Magnetic Properties of a Complex Ion - Paramagnetic vs Diamagnetic
12. How To Calculate The Crystal Field Stabilization Energy
13. The Spectrochemical Series of Ligands
14. How To Predict The Geometry of a Complex Ion
15. Crystal Field Model Geometries - Octahedral, Tetrahedral, Square Planar, and Linear
16. How To Calculate The Wavelength of Light in Nanometers Given The Crystal Field Splitting Energy In Kj/mol
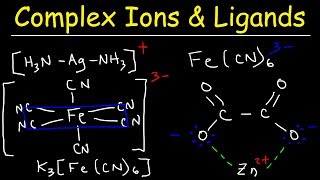







Get The Full 1 Hour Video: www.patreon.com/MathScienceTutor
Direct Link to The Full Video: bit.ly/3vShovS
Chemistry PDF Worksheets: www.video-tutor.net/chemistry-basic-introduction.html
Thank you so much for your content!
Tq
I hope you realize how much we all appreciate these! Thank you so much 😊
Dear chemistry tutor,
I am a chemistry laboratory technician trainee from Berlin. I want appreciate your work and the way you chop every topic into digestible and understandable pieces. I wanted to ask you, wether you´d like to do a video conference with my school and explain us hwo we can learn chemistry easily and show us your methods. you are very inspirational. The chemistry department would appreciate it.
This man is insane. I was just here last week learning about calculus 2 and here I am again learning about crystal field theory
🙌
That man was different. Any other person.
Dislikes from professors who couldn’t explain it this easily.
😂😂
That’s why I’m here
Ekdam Sahi❤️
What? 💀
Low brain
You dont even need college. All you neeed is this guys videos and its much better than college lecture slides. Thank you so much. Your videos are so well explained. Awesome
Facts. Literally we are out here paying college tuition to lecturers that just leave us confused, when Organic Chem Tutor deserves all our tuition and explains things under an hour. He is a legend!
Literally my situation , paid so much for the lectures , but all the lecturer does is read , does practices where she skips all the steps , and expects us to just know the content and then throw hw and exams and tests , like wth . This dude is saving my life
I have to say this man ,it's been a hell of a year for me you know first year of chemical engineering...and i got to tell you how much I'm appreciate what you doing I'm really glad that i found your channel ,you've helped me much more than my college did ,you just simplified anything and this made me suffer much less , if i could i would've donate more ,glad you here man :).
bruh .. u learning this at chemical engineering ????!!!!!!!!!!!!,... we are having this as our 12th std topic ☠☠
@@Parakeetboy_yt exactly 💀
@@Parakeetboy_ytyou'll get those again in engineering
@@Parakeetboy_yt same. INDIA
@@Parakeetboy_yt9 th class
Your videos are always the first one I click on when I search up a difficult concept such as CFT, thank you man!
Thank you so much for this! I was struggling to understand this since we just went over an entire chapter in 40 minutes and have a test the day after tomorrow on it. Your way of explaining stuff is amazing. Thank you once again!
We really appreciate your efforts ❤️
this man explained what my professor couldnt explain in just 20 min. legend
Thank you so much for this amazing tutorial video!! You’re the greatest professor I’ve ever seen!! I’ll be writing my inorganic exams late next month, and I’m assured of an excellent performance due to your help. May God continue showering his abundant blessings upon you! 🙏
Just small correction: d(x^2-y^2) is on the X Y axis, and d(XY) is between the axis.
ahh yes i got confused thinking i was wrong thank u!!!
This confused me too! So much easier to remember the squared ones together and was afraid that didn't work
Yes, I checked in the comments section to see if anyone pointed this out...
Thank you so much for all these videos. It is providing more help than you think.
I'm writting my exam tomorrow, this video was better then my 3hour×5 lectures.
i really enjoy how you teach these information !!! god bless you real professor
my prof zoom link he took me over 50 mins to explain the crystal field theory, but I couldn't understand it. But you guy do it. Thank you so much.
I need your help
Yes ! I love this video. Could you do some more inorganic chemistry videos and along with formal mathematics?
ruclips.net/video/ihoypNO3L3M/видео.html
I finally got it.... thank you! ♥️
Professor you have helped me understand Crystal field Theory, well done. I really appreciate your effort
holy moly dude, where have you been? thankyou so much xx
😊What A clear Explanation!!!!! Loved It ❤
thank you for explaining it in such a easiest way
Appreciate your vids
you have a vid for everything man, thank you thank you thank you
Thank you so much for this!
you helped us alot thank you brother
Thanks, your videos are pretty useful ❤
Thank you so much this video was of great help
This man can teach me something in 20 minutes that took my professor a week.
You are great man.Thanks 👍
can anyone please tell me which one is more stable, Fe2+ or Fe3+ on the basis of octahedral distortion? please
You deserve the best man 😎
thx for explanation much love!!!
Thank you so much
Thank you!
Please also do molecular orbital for octahedral complexs
Thanku Happy New Year🤗👍🥳
Nice explanation
Very nice
you just save my chem exam
I am so grateful
hwo do we know now tho which field applies to the complex we are naming? do we just look which orbital arrangement filled with the electrons is the most stable?
It was smooth 😎.
If I study chemistry from you I think I will fall in love with it 😂.
You don't need a perfect drawing because it's those perfect drawings that sometimes confuse the hell out of me.
Thank you so much!
You are a lifesaver!!
ruclips.net/video/ihoypNO3L3M/видео.html
Thank you greatly.
Thanks very much!!!
Is this a person or a computer? Whatever it's, it's too good
Well explained, I am almost 🎓, keep up good work
You're a life saver
YOU ARE A KINGGGGGGGGG
I am from India and last in last year of my high school I find this video very helpful thankyou.
That was great. Thanks
ruclips.net/video/ihoypNO3L3M/видео.html
YOU ARE THE BEST OF THE BEST
I noticed that he handles the axes quite loosely during his drawings of the d orbitals. The xy, xz, and yz orbitals are off axis, while x2-y2 is along the axes as well as z2 being along the z axis.
thank you so much!
Best ever,,bro deserve hon.nobe world prize
You are Amazing.
Excellent!!
Tell me if i'm wrong, but shouldn't the PE in the final exercise be equal to zero. This because you only add P for electrons that pair due to the pairings energy being smaller than going into the eg levels and therefore you add it, otherwise you should have added the +3/5 delta oct. Therefore with high spin, there is never PE involved and for low spin for PE, you only count the pairs that are formed that would not have formed if it had been a low spin complex.
This is exactly what i wanted to point out. As you say, you only count the pairing that ocurrs in comparison with the hypothetical situation of espherical charge surrounding the metal, when all d orbitals are degenerate. That is because the crystal field stabilization energy is the relative stabilization of the complex vs. the ideal situation where no splitting of the orbitals takes place. This means that, for a d6 in an espherical charge distribution situation you already have one electron paired, so the crystal field stabilization energy for a high spin complex doesnt involve electron pairing.
@@sonemaster yes you are right
Thanks a lot👍
yay! please make more inorganic videos
Tysm man
Great video! Just one question: I thought the dx2-y2 orbitals are parallel to the x and y axis?
i believe parallel to the plane of x and y and within the actual x and y axis
@@gabrielhigginbotham7101 Yeah. In the video, he explains it in a way that the dx2-y2 orbital isn’t directly along the x axis and y axis. Now I’m so confused…
@@crevect4799 but he literally drew them along the x and y axis bruh
I was confused about this too, he said that x2y2 is not on the axes and that xy is on the axes.. although it seems that he might have mixed them up? I can only find the orientation with x2y2 on the axes
Thankssss❤❤❤❤
The pairing energy contributions to the CFSE are done incorrectly here. The CFSE is the stabilization energy of the d electrons due to the energy splitting of the d orbitals. So, for both the high-spin and low-spin (weak- and strong-field) cases, the comparison should be with a system with zero splitting of the d-orbitals. For a zero-splitting case, there would still be one orbital with two electrons when there is a total of 6 d electrons. Therefore, the CFSE for the d^6 high-spin system does NOT include any pairing energy contribution because that electron pair would still be paired even if the d orbitals weren’t split at all. And the low-spin case would include only 2P, not 3P - again, because one electron pair would still be paired even if the d orbitals weren’t split at all.
Happy new year sir
ruclips.net/video/ihoypNO3L3M/видео.html
At 20:05, the pairing energy is included in the CFSE calculation, even though there would be an equal pairing energy in the original d energy level (as in, prior to crystal field splitting). Therefore, should the pairing energy really be included in the CFSE calculation?
yes
Hello. Can anyone please help. How does CFT account for the colour of transition metals? ?
Basically, transitions between the t2g and eg levels are responsible for the colours the metal complexes show. The energy gap between the levels depends on the metal and the ligands.
hey why are the units of cfse in cm-1
o nghakolla blind ko bolelle khutmane. big ups👍👍
Thanks
what electron configuration do you need to have to be a strong field?
fields are dependent on the ligand and is given by the spectrochemical series. ligands like ammonia, water and carbon monoxide are strong field ligands and the halogen anions are weak field ligands
Hellow sir,
Could please explain about tetrahedral and square plannar?
Sir I have doubt in d orbital shape
I donno he explains so perfectly
Like I blindly just listen to him
Thanks to him I completely so many topics for neet
May God bless you
Thanks a ton 🥺🌼✨
Can you please explain the coordination number 4 complexes also
i rlly hope ur having an amazing day
We have to learn this in high school, this is so frustrating
Can you please explain walsh diagram
The plane-orientation of dx2-y2 and dxy is probably mixed up in this video?
dx2-y2 is on the axes and dxy is between is my previous understanding
Thank you kind sir. My inorganic professor's lecture notes are a fragmented disaster XD
How do you know if it is weak field or strong field?
From the streptochemical series
Nice
you guys are learning this in college?
Yes
only 13k views?
Must be really easy ;)
thanks lot ur Savior
But this energy distribution after splitting is only for octahedral config if there's a tetrahedral config then the distribution is totally opposite
kinda sad not the whole thing is there but pog vid thanks bruh
Hi . Please we need a lesson about glycosylation of protein . Thnx
Good
dx²-y² is directly on xy axis and dxy is in the XY plane but not directly on x and y axes
FANTASTIC!!!
u saved me
btw it's Delta sub o not sub 0. The o stands for octahedron
This guy is literally saving us from being weeded😭, we survive another year in college, sigh🚶♀️
I thought x^2-y^2 was right on the axes, and the ligands are also right on the x, y, and z axes, which is why x^2-y^2 is unstable (as well as z^2)?? I think you taught the opposite..
I think he misspoke a bit but still came to the right conclusion
14:50
wont that violate pauli exclusion principle
GOAT