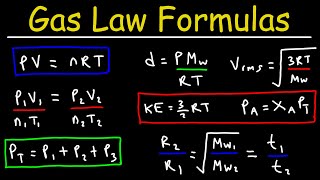
Ideal Gas Law Physics Problems With Boltzmann's Constant
HTML-код
- Опубликовано: 24 ноя 2017
- This physics video tutorial explains how to solve ideal gas law problems especially using Boltzmann's constant. This video contains plenty of examples and practice problems.
Boyle's Law Practice Problems: • Boyle's Law Practice P...
How Does a Bike Pump Work?
• How Does a Bike Pump W...
Charles Law:
• Charles' Law
Gay Lussac's Law:
• Gay Lussac's Law Pract...
Avogadro's Law:
• Avogadro's law Practic...
_____________________________
Translational Kinetic Energy:
• How To Calculate The A...
Molecular Speed of Gases:
• Molecular Speed of Gas...
Mean Free Path and Free Time:
• Mean Free Path, Mean F...
RMS vs Average Speed:
• Root Mean Square Speed...
Molar Heat Capacities of Gases:
• Molar Heat Capacities ...
Phase Diagrams of H2O & CO2:
• Phase Diagrams of Wate...
____________________________
Relative Humidity & Dew Point:
• Relative Humidity - De...
Fick's Law of Diffusion:
• Fick's Law of Diffusio...
Open Vs Closed Vs Isolated System:
• Open System, Closed Sy...
First Law of Thermodynamics:
• First Law of Thermodyn...
Final Exams and Video Playlists:
www.video-tutor.net/
Physics PDF Worksheets:
www.video-tutor.net/physics-b...








Final Exams and Video Playlists: www.video-tutor.net/
Full-Length Math & Science Videos: www.patreon.com/mathsciencetutor/collections
thanks for the R and k relation! was ALWAYS confused at that.
Phsyics would be so goddamn easier if it didn’t have conversions
Bruhh u good?
A couple of days ago the Ideal Gas videos were all apart of your physics playlist but now they are gone. Where can I find these videos?
I know this is a hella old video and not sure if anyone will even see this but can anyone explain why my Homework portal from my school has the answer in suggesting *answer box to input answer* "10^23" ???? All my answers are coming out to "10^24" I've triple checked my computations and followed those steps in the last problem to the tee. Even online HW resources have their answers in "10^24" but the portal is having "10^23" next to their answer box
thank you
your the man of the hour holmes
I think if we find n value first and multiply it with Avogadro number, it will directly give number of molecules. I have cross checked the answer value and it's the same. Please let me know what you think.
thats exactly what I found as well, and it is also how my physics professor taught me how to do it. I think it is correct in this case, and in any other case where you have similar units.
How can you use Boltzmann equation to find approximate population of an excited state?
I don't know if anyone's gonna see this but please if you know, can you answer my question?
Why did he use PV=NKT in that last example? Thank you so much!
because it asked for number of molecules instead of number of moles, so if you used pv=nRT it wouldn’t of worked because that finds moles. so you simply should remember this equation for finding any question that needs you to find molecules
when are we supposed to use R vs. K?
They both ultimately are the same thing. It's just that R is expressed in terms of moles, and k (usually lowercase k) is expressed in terms of atoms or molecules. One is a macroscopic unit, the other is a microscopic unit. Moles ultimately are just a convenient counting unit called "the chemist's dozen", so it makes it practical to express populations of molecules without scientific notation.
Good
STP according to IUPAC is 273.15 K and 1 bar (100 kPa). NIST uses NTP and that is 293.15 K and 1 atm.
The IUPAC standard for STP changed in 1982, so it's possible he got his definition of STP from a textbook printed in the '70's, which would use 273.15K and 1 atm, instead of 1 bar.
I don’t know, today I know my teacher still tells us to use 273.15k
That's very interesting
10,000 mL is equal to 1 cm^3 not 1 mL
Hi
👌🏽🏆
Isn't standard temperature 298k?
nope its 273 K.
@@robertowisconetti2732 but isn't that 0 degrees? In the UK our spec says 25 degrees (298k) might be different for you guys though
@@kiransteward5387in celsius its 0. converted to k its 273. 25 degrees is the room temperature. u have mixed da facts. if u need any help. i ll help ya
Wespuchchi Nasarawo it’s 273.15 K = 0 degrees Celsius.
@@neilbergin3835 yeah I know that but in chemistry we take 298k and 100kpa as standard conditions under the topic of enthalpy changes
k
Good