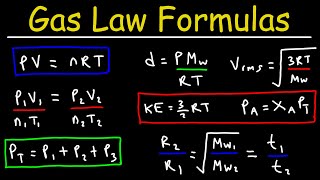
Combined Gas Law Problems
HTML-код
- Опубликовано: 26 сен 2024
- This chemistry video tutorial explains how to solve combined gas law problems. This video contains many examples with all of the formulas and equations that are needed.
Pressure & Boiling Point: • Introduction to Pressu...
Gas Pressure Unit Conversion:
• Gas Pressure Unit Conv...
Manometers & Barometers:
• Manometer Pressure Pro...
Water Height & Mercury Column:
• Height of Water in a B...
Boyle's Law Practice Problems:
• Boyle's Law Practice P...
_________________________________
How Does a Bike Pump Work?
• How Does a Bike Pump W...
Charles Law:
• Charles' Law
Gay Lussac's Law:
• Gay Lussac's Law Pract...
Avogadro's Law:
• Avogadro's law Practic...
Ideal Gas Law Problems:
• Ideal Gas Law Practice...
Combined Gas Law Problems:
• Combined Gas Law Problems
_______________________________
Gas Stoichiometry Problems:
• Gas Stoichiometry Prob...
Molar Mass of a Gas at STP:
• Molar Mass of a Gas at...
Gas Density at STP:
• Gas Density and Molar ...
Dalton's Law of Partial Pressure:
• Dalton's Law of Partia...
Collecting Gas Over Water:
• Collecting Gas Over Wa...
_______________________________
Gas Density of Mixtures:
• Gas Density & Average ...
Average Kinetic Energy of a Gas:
• Average Kinetic Energy...
Graham's Law of Effusion:
• Graham's Law of Effusion
Kinetic Molecular Theory of Gases:
• Kinetic Molecular Theo...
Gas Law Problems Review:
• Gas Law Problems Combi...
_________________________________
Final Exams and Video Playlists:
www.video-tuto...
Full-Length Videos and Worksheets:
/ collections








Final Exams and Video Playlists: www.video-tutor.net/
My chem teacher complicated this so much. Thankyou for explaining it clearly
My chem teacher didn't even explain it lol he just gave us the formula and called it a day lolol
@@nah_. lol
I always wonder why your channel is called the Organic Chem tutor. Cause you do fantastic job at explaining every subjects.
Not really
@@twinkjakdoomer what
Or maybe cause he teaches organic chemistry
you always explain it very well man, the best narrator in all tutorials
Thanks
Professor Organic Chemistry Tutor, thank you for explaining How to solve Combined Gas Law Problems in AP/General Chemistry. The derivation of the Gas Laws along with the practice problems really increased my understanding of the material. This is an error free video/lecture on RUclips TV with the Organic Chemistry Tutor.
I may have only watched from 4:00-4:30 but I now completely understand the topic
thanks king
You teach better than my chemistry teacher pls keep it up you're the reason I'm passing
Thank you so much for this! I was lost with the combined formula and you made it so easy!
If you existed year 2003 , for sure I have high grades in College. I love the way you present ( detailed, and has a calm voice)
Bro saved me from failing chem, that shit went from F to A real fast 💀
You saved me no cap I understand this concept so much better
God bless you bro❤️. Ty
This is one of the only not perfectly explained video
When it comes to the first example, what if you couldn’t clearly see that something was divided by an obvious value?
I'm a freshman in college as a Chemistry and Botany double major and let me tell you that the way you explain the rules and concepts is much clearer than most of my professors! Thank You so much!
Bro, you helped me so much. Thank you!
I'm paying 18k per semester just to watch RUclips tutorial
Excellent explanation 🙌
New title acquired: God of Explanation🎉🎉🎉🎉🎉🎉
Currently passing chemistry thanks to you. 😄
I hate it when i didn't understand it but i love it when im solving it
Big thanks as always!
How about if the problem was asking about the temperature? The question number 1 is finding about the p2 and in number 2 is the same also .. so how we gonna do if the temperature was asked in the question?
How do you find the second temperature in a problemmm
Thank you you are very helpful
Thank you so much, my textbook makes no sense.
so do mine TwT
Thanks man you've really made it extremely easy for me
This is what I call a teacher . . . better yet, a professor.
❤😂😂😂 the way you derived the combined gas law 👏 🙌 🙏 👌
I hate math
Same fuck math
It’s chemistry
@@Missuuushsdhhd its still math in it, caculation
Hope your narrative has changed
It's a year
I love Math
For any curious mind
Think positive
A process consist of two steps: (1) one mole of air at T = 550°c & P = 3.5 bar is cooled at constant volume of T = 80°c. (2) The air is then heated at constant pressure until its temperature reaches 550°c. if these two step process is replaced by a single isothermal expansion of the air from 550°c & 3.5 bar to some final pressure P, what is the value of P that makes the work of the two processes the same? assume mechanical reversibility and treat air as an ideal gas with:
Cv = 3/2 R & Cp = 5/2 R
how to answer this? please teach me
these videos are why i'm passing science
need help po
A gas sample at a certain pressure and absolute temperature occupies a volume 0.5 L. If the temperature is doubled and the pressure is reduced to one-third of its original value, what is the final volume occupied by the gas?
This is easy. I lub you chem tutor
I lub his vids too 😂
About 1:29 I was panicking because my book shows constant moles
this is fantastic thank you.
Thank you so much
can you used this formula; V= nRT ?
great explanation. definitely made things easier.
this guy taught me all the gas laws in 50 minutes, when my teacher couldnt do it in 2 months
What if P2 T1 and V1 are only given?
If we have only one pressure,mole and temperature how can we find p2??
Can you use ideal gas law on the last problem? which has mol?
I'm thinking the same thing
Can someone tell me why did the he times 9,i don’t understand
Hey, I have a question please can someone help me. I have been watching gas law tutorial online cause obviously, I can't understand my teacher. Then I have learn that you should convert Celsius to Kelvin. But she continue to solve our problems using Celsius. I don't really understand
Maybe your teacher is using Delta T in the Questions | 8:00
ur single handedly holding up my chemistry grade rn 😭
hello I am confused on a problem on my homework, the asked was "what is its volume at STP?" my question is, is the volume asked the v1 or is it the v2? please someone answer me thank you.
at stp, the volume of a gas is 22.4L
You should start your own college
Can i already convert torr into atm because it just gives me a longer time to calculate
Divide the torr by 760
not sure if im underthinking number 2,but for that question could you not just use pv=nrt.
Andrew Buchtan no, it has to say for example assumed at ideal gas laws. Usually problems will say that, because if u use ideal gas law equation you have to plug in the constant of r which will give u an entirely different answer
No, you can not only use the ideal gas law because there is more than one volume number to add to the equation.
Is this combined gas law or ideal gas law?
Super 😉
Gosh you're the best
hi! how n2 is 1,9 mol, it is sayed n2 is 1,25 mol?
I really appreciate this. Thank you!
Wait a minute this is ideal gas law
They isn't different
the second one
Same thing just in a different form
This is good.
Let's make this video reach 1K likes like the other ones
you mean... AhEm ...... 1.2k likes UWU
I’m still in middle school and I already have gas laws😢😅
Frick yeah. I did it.
Good
11:42 bro we dont get calculators in india
you’re healing my daddy issues
yawa ning chemistry
Love u g
You forgot to tell us how you converted it to atm, somebody pls tell me how
actually torr to atm is torr/760 and atm to torr is atm*760
I'm just studying for my test :|
Online learning fucking sucks
wowwwwww you complicated this so much more than my professor.... Im actually more confused now....
we didnt even learn this and we had this on our hw
Same
JOHN 3:16
FOR GOD SO LOVE THE WORLD, THAT HE GAVE HIS ONLY BEGOTTEN SON, THAT WHOSOEVER BELIEVETH IN HIM SHALL NOT PERISH, BUT HAVE EVERLASTING LIFE
I hate chemistry and todays my birthday 💔
This video is great on mute
Big help thank you
I would buy your merchandise if they had different designs or even just said "organic chemistry tutor" instead
The add I got for your video should be illegal
thank u for helping me pass AP chem
i hate avogagos law
Excellent explanation 🙌