How To Calculate The Average Translational Kinetic Energy of Molecules Using Boltzmann's Constant
HTML-код
- Опубликовано: 5 сен 2024
- This physics video tutorial explains how to calculate the average translational kinetic energy of molecules using Boltzmann's constant. It also discusses how to calculate the average kinetic energy of multiple moles of molecules using another formula.
Boyle's Law Practice Problems:
• Boyle's Law Practice P...
How Does a Bike Pump Work?
• How Does a Bike Pump W...
Charles Law:
• Charles' Law
Gay Lussac's Law:
• Gay Lussac's Law Pract...
Avogadro's Law:
• Avogadro's law Practic...
Ideal Gas Law Problems:
• Ideal Gas Law Physics ...
_____________________________
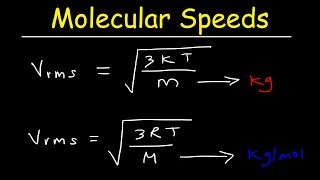
Molecular Speed of Gases:
• Molecular Speed of Gas...
Mean Free Path and Free Time:
• Mean Free Path, Mean F...
RMS vs Average Speed:
• Root Mean Square Speed...
Molar Heat Capacities of Gases:
• Molar Heat Capacities ...
Phase Diagrams of H2O & CO2:
• Phase Diagrams of Wate...
____________________________
Relative Humidity & Dew Point:
• Relative Humidity - De...
Fick's Law of Diffusion:
• Fick's Law of Diffusio...
Open Vs Closed Vs Isolated System:
• Open System, Closed Sy...
First Law of Thermodynamics:
• First Law of Thermodyn...
Final Exams and Video Playlists:
www.video-tuto...
Physics PDF Worksheets:
www.video-tuto...








Final Exams and Video Playlists: www.video-tutor.net/
Full-Length Math & Science Videos: www.patreon.com/mathsciencetutor/collections
saahi tha bhai gr8 explanation
May I know why for the first question we don’t use KE=3/2NkT but instead KE=3/2kT only?
Because the formula represents the average kinetic energy per molecule if you wanted to find the average kinetic energy of 1 molecule then you would have to multiply the average kinetic energy per molecule times 1 molecule
Thanks. Pretty simple and easy to understand explanation.
Can you do the videos showing translational and rotational kinetic energy , for it hard to demonstrate in class.
Really good!
Channel is underrated 🔥🔥❤️🔥
3 million subs...
He has millions of subs and this nigga sayin he's underrated 💀
😂😂😂
Vaaipilla Raja.......................🎉
Thank you so much sir ❤
You're the best
How Do You Get The 1.38???
boltzmann constant
is it always 3/2.. ? or it changes when the level of freedom changes? (like 5/2..)
how did you get R?
r is gas constant
@@MonkeyDKizu yes
Yes
gas constant is something like 8.31 KJ correct me if I am wrong
@@user-ui9kt3fv2n 8.314 J..
tysm, that was very clear!
the average translational kinetic energy ( KE = 3 RT/2 Na) and the average kinetic energy ( KE = 3RT/2) make me really confuse . can u explain for me :(((
Avg translational K.E =(3/2Na)KT ..but this is for 1 molecule
And avg K.E =(3/2)RT....this is for one mole..so don't get confused
you are god
great
Like!!!!
🥶