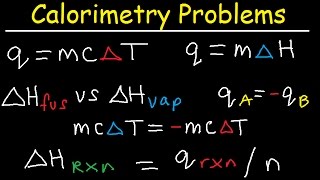
Latent Heat of Fusion and Vaporization, Specific Heat Capacity & Calorimetry - Physics
HTML-код
- Опубликовано: 26 сен 2024
- This physics video tutorial explains how to solve problems associated with the latent heat of fusion of ice and the latent heat of vaporization of ice. It contains problems associated with specific heat capacity, calorimetry, and heat transfer problems. It discusses the heating curve of water, the increase of kinetic energy during a temperature change process and the increase in potential energy during the phase change process. This video contains plenty of examples and heat transfer practice problems. It discusses phase changes such as freezing, melting, vaporization, condensation and the heat exchange that occurs during those times.
Linear Expansion of Solids:
• Linear Expansion of So...
Temperature Conversions:
• Celsius to Fahrenheit ...
Thermal Stress and Strain:
• Thermal Stress and Str...
Heat Energy - Unit Conversions:
• Joules, Food Calories,...
Molar Heat Capacity:
• Molar Heat Capacity Pr...
Calorimetry Problems:
• How To Solve Basic Cal...
_______________________________
Specific Heat Capacity Problems:
• Specific Heat Capacity...
Final Temperature Calorimetry Problems:
• Final Temperature Calo...
Conduction, Convection, and Radiation:
• Heat Transfer - Conduc...
Heat Current and Temperature Gradient:
• Heat Current, Temperat...
Thermal Conductivity and Stefan Boltzmann's Law:
• Thermal Conductivity, ...
______________________________
Boyle's Law Practice Problems:
• Boyle's Law Practice P...
How Does a Bike Pump Work?
• How Does a Bike Pump W...
Charles Law:
• Charles' Law
Gay Lussac's Law:
• Gay Lussac's Law Pract...
Final Exams and Video Playlists:
www.video-tuto...
Physics PDF Worksheets:
www.video-tuto...








Final Exams and Video Playlists: www.video-tutor.net/
you've covered a 3 days lecture in 3 seconds and explained it way better than my lecturer ever could, thank you.
a 30min video in 3sec?
true
@Let's Make gotta love storytime
You must have ADHD.
3 days ? I took it in 2 hours only😂
*cries* you're basically my physics teacher right now. ily.
Edit: I’ve graduated high school, you guys can do it!! I believe in youuu
then you my classmate. We're in the same boat
@@najeaandfriends6183 I wish you luck friend.
All true, y'all my classmates
Hey wanna skip class my classmatesss
Au di yes mr. Classmate lets skip today
seriously bro no intro no outro just straight to the point in the most simple wording possible
Bruh how tf did I learn more here in 20 mins than 8 hours of being taught by an actual teacher. Actual Legend man, Your my new physics teacher lol
In the final problem you made a mistake. The change in T for Q(h2o) should be 27-21 not 27-20 and the answer should be 127 J/KG*C
You deserve more likes.
Suggestion plzz
127.5 to be precise
I've tried to do this problem for more than 3 times getting that answer thinking there's something I'm not doing right
@@yashjiwani1040 more like 127.4390244
dude, I walked out of Chem II totally vibe checked, but you have somehow risen me from the dead in 31 minutes. THANK YOU!
I'm in university physics for life sciences 1 and you're continuing to save my life just like you did last year to get me into university! thank you for your dedication
you're literally carrying my grades in three of my subjects dude, thank you
For every chapter, my teacher provides support videos to help with the topic; your videos have made numerous appearances and for this unit, your videos are our introduction. I use your videos mainly for calc. All of my calc 2 notes come from your videos. Everyone comments how amazing you are but you truly are carrying America's engineers through their general education courses. The impact you have made will be around for decades to come.
in IB physics rn and this literally covers 50% of topic 3. Thank you sir
me too :D
ikr
Im currently in hl physics and i am failing pls send tips
@@rico-enI get you… currently preparing for my SL mock that’ll happen in few hours-
@@nevermind9835goodluck i just finished my final exams last week just hang in there❤
Physics final tomorrow morning. Thank man!
Same
Me too.
Me three
What a legend sia, for some reason, all i needed was to watch like the first few minutes of the video to have a greater understanding of latent heat as compared to when im in school learning this for at least 2hrs
TY SO MUCH TEACH
Oh! Wow! this method of explaining is so amazing and very easy to understand. Teachers should explain like you. Thank you so much. 🙏🏼🙏🏼🙏🏼🙏🏼🙏🏼
this video was so incredibly helpful, I understood everything after being lost for weeks in class. THANK U SO MUCH
Thank you very much!!!! Your videos are so clear and easy to understand, especially with the examples that you show. I was studying for my final physics exam and I was confused on many topics but I then started learning with your videos and ended up getting an A! Thank you, thank you, thank you! Could you do some more videos based on General Physics 2? Thank you and hope you are safe during this pandemic!
Do u know how to do specific heat or heat of vaporization and heat of fusion I need help
@@alishaislam9275 Sorry I have seen this now
I can't thank you enough for explaining things in such a way that my professor can't.
this made so much more sense than the book and the teacher combined. Thank you
Q=ML -use whenever u have a phase change
Q=mcAT -use whenever the temperature change
I can't thank this guy enough.
Your videos helped me through college and continue to help me through my career, thanks!
Well done boss, my lecturer at UEW didn't help us at all
i have no words, keep up the good work, it would be an understatement to say that you're amazing.
Made me understand a whole section within few minutes...I appreciate your standard of explanation
You helped through highschool and college. I can't thank you enough. I wish you'd do more bio/med videos. I'd pay to see you explain these concepts😍🙏
Bruh you just changed the whole day tutor into just 30min, thank you very much 👏👍👌
THANK YOU SIR FOR YOUR EFFORTS IN ASSISTING THE WORLD WITH PHYSICS I M FROM SOUTH AFRICA BY THE WAY, BUT AS I WAS DOING THE PROBLEM ON MY OWN I DISCOVERED THAT THE ANSWER IS 127.6 C . I THINK THE MISTAKE ON YOUR PART OCCURRED WHEN YOU WERE DETERMINING DELTA T , INSTEAD OF INITIAL TEMP 21 YOU HAD 20 DEGREES CELSIUS
Bungane Radebe damn man why u gotta shout like that
Nigga be quiet
@@donnaa7640 damn Donna chiiiiiiill
WHY ARE YOU YELLING
When talking about the green house effect, people count vegetation as a dark material but vegetation is sculpturallly complicated, so that some bits of leaf are in the light and many others in shade so that they are often categorised as dark and so as collectors of heat not reflectors of visible light, when all sunlight falling on the reflective top of leaves get reflected back up skywards.
If you are taking a photograph of leaves, the pools of light on the lit bit of the leaf annoyingly come out as a stronger shape than the actual leaf comes out as being, the shine coming off leaves often often comes out as light, as bits of white in the photograph it comes out as white. If you take a photo of the sunny side of a tree, its colour is much lighter than the other side but even this lighter side does not relfect the shinyness of leaves because on the sunny side of a plant you see the underside and so the non sunny side of the leaves that are above the camara and as leaves are often curved you also see the sides of leaves that are dark because curved away from the sun. I think all this should be mentioned when people categorise the effect of vegetation on climate change.
Another aspect of the usefulness of vegetation as a coooler of the world, without mentioning how they take carbondioxide out of the air, is how very shinny hay and straw are, so this dead vegetation would, if left on the ground, both aislate what is a pretty good material as far as storing heat is concerned, earth, i have read that earth has the same ability to store heat as brick but also, hay and straw are very reflective grasses are full of glass, silica, which should put animals off eating grass, so hay and strawlike live leaves and probably more so, also reflect sun light back towards space.
Have you thought how much land world over is left fallow for a year where wheat is grown? Half as much as is used to grow wheat, though this is not an agricultural habit used in modern Norht America? Also lots of land is ploughed in spring, just before the heat of summer, in preparation for autumn planting of wheat so leaving so much earth to accumulate heat, that it is absolultely mind boogling.
once it did nto matter how much of a material which was a good accumulater of heat was left bare to be heated by the sun but now things have changed and it does matter.
You explained it better than my lecturer did thanx
30:58 the given initial temp for water is 21 degrees not 20 degrees. Thanks for the vid anyway, very helpful!
final answer is 127.62 J/kg C
I appreciate your videos in every universe.
Thank you so much for this vid. Now I undestand it well but there's a tiny mistake you made on the last problem. You wrote 27-20 instead of 27-21. Thank you soo much.
Right on time! My exam is tomorrow night! Thanks
Keke Wilson ME TOO FROM FUTURE
Same lol
5y🙃🙌🏻
Physics is getting real in me waoo, thanks 👍
Thank you sm. I’m in Honors Physics and we’ve just begun a very difficult lab involving fusion. Thank you!
Thanks a lot!!! Took Months trying to grasp all these but Got it all in 31 min.
u have just increased my knowledge..... Thank you
You deserve my teachers position
Thank you so much my guy,,,u helped me so much is the confusion I had on thermodynamics.... thanks thanks thanks God bless u....I took some weeks trying to understand this but u helped me in 31 minutes,,, thanks my guy
I'm writing exams tomorrow and this really helped because this is exactly what I was looking for😀☺👓👍👏👏👏👏
bruh you are the best tutor on the planet
I love youuuuu ❤️❤️❤️ thank you , you have no I idea how much you have help me out with physics
Thank you very much for this video your explanations are always the best Sir.
I think you made mistake at 29:50, final temperature of the water according to the given statement is 21 degrees Celsius not 20 therefore final answer of the problem is 127.6 not 148.9
the final answer for the specific heat of the metal is 127.6 J/kg*c , the mistake that you got at the end is the inital temp for H2O it is 21*c
As I noticed too
Man your teaching is the best
You teach so well tho , keep the ball rolling
I watched you in high school, now I am in collage and watching you
So you’re majoring in art?? lol
@@derekchatcavage9180 No I'm in betrol institute
@@Gmaing-madrataIs Arabic your native language? A lot of English jokes are very confusing to non native English speakers. For instance, you spelled college wrong. You spelled it as collage. In English a collage is a type of artwork. So I was trying to make a clever joke around it…
Hope that makes sense, not trying to be rude. One love brother!
26:48 for this question i got 24,094,500 J
Will be taking my exam few hrs from now, thank uu!
This was very helpful now ill pass my exam lol
saaaaaame lol
well explained,indeed,with ur help i managed to answer all my tut questions
you have singlehandedly saved my school year lmao
I wished that this was recommended to me earlier before I took my trials ;-;
I owe this guy my life
Even at this time the video is very important 🎉Thanks man.Your didna great job.🎉🎉
I like he says the word earth, "Earf". makes him even more badass.
thank you so much for the explanation but there is a problem in the last question. you made a mistake somewhere at 27:02 if you check your calculations, it is heat gained by hot metal= heat gained by water
but the problem is that when getting your temperature difference, you wrote (27-150) which is wrong. it is (150-27) because heat is being lost final temperature- temperature of the metal itself.
Yeah your the reason i passed my chemistry test
Thank you so much! I understand this because of you!!
Your explanation is so clear. Thanks!
Well Thank you your videos helped me a lot I only had hours to study due to writing multiple exams
You are simply the best
WOW you explanation is perfect but in the last problem about the metal and water , in heat of water you compensated the change in temperature 7 but it should be 6
Explain Latent heat of fusion, évaporation. Specific heat capacity, heat capacity and their differences as far Calorimetry is concerned
29:50 isn't it supposed to be 27-21 not 27-20 since the water's initial temperature is 21 C as given in the problem?
Thanks man ❤. You are always straight to point 😊
Awesome explanation dude
Thank you a lot may the almighty bless you thanks a lot ...now am good 👍 at this topic forever
this man is a legend
you help me better than my teacher ever did
My Physics improved from 50% on semester test 1 to 97% on semester test 2🎉🎉
Thank you sir, I have nothing to offer. I’ll thank you properly in the future.
Love you man, saved me so much
Bro for real, thank you so much fmd.
Thank you very much you have helped me alot
I hope you're my lecturer!
In a physics class, the instructor has assigned a task to determine an experimental value for the heat of fusion of ice. The students dry and mass out 26.8-gram of ice and place it into a coffee cup with 100.0 g of water at 36.4°C. They place a lid on the coffee cup and insert a thermometer. After several minutes, the ice has completely melted and the water temperature has lowered to 18.1°C. What is their experimental value for the specific heat of fusion of ice? What is the answer?
Wonderful video
in the calorimeter question you use 27-20c for the water. should it not be 27-21c or am I missing something?
😀😀 I also thought I was the one wrong
Yes you are right. where he put 20, it should be 21. He's human like the rest of us. It doesn't matter how good you are or how smart, mistakes will always be inevitable which is why you can't always take his work as law.
For example he seems to like working with C and L in joules, where as my textbook has them in KJ with the decimal moved to reflect it. I've been using my numbers and so my answers have been tending to be a little more accurate then his just because of the decimal placement. Sure enough when he converts back to KJ at the end, our answers are very close.
Very Helpful. Thankyou 😁
Thank you so much, this lecture really helped me so much😭
I have a final in 30 mins and I’m studying this now lollllllll I’m about to shit myself
How can the ice be a water at 0celecius? It’s wrong (10:00min) for that exercise to covert the ice to water at different temperature we have two kinds of heat which are q=ml and a=mcdt just added them
Thank you so much! This was very helpful!
Dude. The more you learn, I didn't know that the temperature stays at 0 and 100 for some times until some spesific time period. It's whacky
but why exactly are we considering Qh as a negative value at 28:00, and how did you deduce the temperature changes at 29:30
Nice video, very good explanation 👍
Hello, just for clarification. @6:40 example, why not include the sensible heat computation since the process required energy before melting the ice to latent heat?
I think on the final problem you distributed your negative incorrectly for waters mcat value. q1+q2=0 so q1= (-q2) the negative is distributed over M, C, and Delta T. I get a specific heat of around 127.62 J/kg*degrees C. when I plug this specific heat value into q1+q2 I get around 0 (0.06=3767.4+(-3767.34)) and the values are rounded so that makes sense. If you plug in 148.9 to the metals specific heat you get the equation 0=3767.4+(-4395.528) which cant be. If i've made a mistake my bad
Great work. Your videos have been helping me. Thanks
There was a mistake in the final question. The initial temperature is 21 not 20. But good work though and thanks, the video was a lot helpful 👍💯
thanks
Thank you soo much for this !!!!
You're a legend
Currently cramming for my year 12 Physics test🙏🏻
Bless you Sir, you have made me understand this in the last minute to my test...
You are simply super amazing, even on the topic of the mechanisms of heat transfer you were great!!
For the last question isn't it supposed to be 27C - 21C? Because the water's initial temperature was 21C, not 20C.
This was VERY helpful. Thank you
Good job, I really learned
you saved my life
Really good video. Awesome job!