Very Common Mole Questions
HTML-код
- Опубликовано: 9 май 2014
- Here are two very common questions about moles. First: we'll learn how to calculate the mass of a single atoms, answering the question "What is the mass in grams of a single atom of oxygen?" Then, we'll use moles to calculate a mass relationship between two elements, answering the question, "What mass of Mercury in grams has the same number of atoms at 64.2 grams of Calcium?" These two types of mole problems are very common on homework, in textbooks, on quizzes, and particularly on tests and exams.
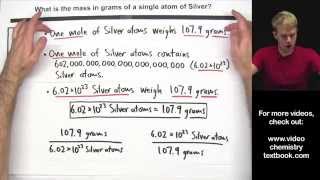








i have just realized why it is so easy to learn from Mr.Dewitt. He patiently repeats connected theories over and over and over; this makes them familiar to me
ye
Just made a youtube account JUST to say THANK YOU. I've watched every video of yours since I started Chem this semester for the first time ever. I got a 91% on my last test!! I'll totally donate to you. Thanks for your videos!! :)
If possible, could you please do more videos like these(with the theme of "very common questions")? Especially since exams are coming up? :P Thanks for your hard work on the videos.
have you graduated, jason? it’s crazy to see comments from so long ago
8 years ago ! wow
how were those exams Jason
yea Jason how were the exams, we really want to know!
This just saved my grade
i was about to drop chemistry because i couldnt understand the way my teacher teaches, she confuses me then she recommended the students to these videos and they are very interested and easy to understand hopefully ill master my next chemistry test
I just wanted to say thank you! literally the only reason why I understood anything in my chemistry exams!
Simply the most simple yet most understandable lectures ever. Sir I owe you one
Sal Khan and Tyler DeWitt both are the best teachers on internet ever I have seen ...
Exactly
I know. They saved my grades.
I wish you were my chemistry professor =(
Man I never seen a better teacher than your teaching style is fantastic
You are an AMAZING teacher. Thank you so much. You have helped me get through my first college chemistry class with a 93 so far and only 4 more weeks to go.
Thanks so much for making these videos! I was really struggling to understand all this, you have explained it simple but very thorough. I think you have saved my chemistry classes and exams!! Great teacher you are :)
your the best chemistry is getting easy for me because of you. my GOD bless you more and more keep it.
This guy is just plain amazing, thanks for helping me through chemistry.
Great video! I was confused about concept of mole and now can understand perfectly. Thanks mate!
Thank you for these videos Tyler. I have a loud class and a quiet, but wonderful, professor in my 8 am chem prep class. I left lecture early so I could watch your vids instead :D.
your the best , i feel so good when i go to chem classes.
explanation is very much better than others: simple to understand
You explain them simply :) I like it
Awesome! Question 1 really clarified why my formulas haven't been working!
you're awesome dude, thank you very much, keep up the good work!
I can watch your videos all day long 👍🏽
Mr Dewitt teaches better than my teacher . expertly explained.
Thanks.
Tyler u r a very good chem teacher
Thanx 4 ur services
This is primeroy from Uganda 🇺🇬
Very very thankful for your videos for giving insights on calculations. Continue to educate us sir.
On that last question, you could just set up proportions. cross multiply.
200.6 x 64.2 / 40.08 = 321 grams of mercury atoms.
Yup, you're totally right! There are a couple different ways you can do it.
+PhillipOnPhire That's what I was going to say too! So, instead of two long steps, you have one. But I guess this shows the whole break up of things and why it's being done, so it makes more sense.
@@tdewitt451 please post more often
@@TPNE99995 he stopped
Awesome videos! Really cool to see you attack the seemingly daunting questions in a way I never would've thought of. Also helpful, as I was stumped for a few minutes, trying to solve the problem like I would algebra! :D Thanks for the awesome explanations!
It's great to see into the mind of a scientist instead of a mathematician ( not that I am one, though) :D
I think this just saved my chemistry grade!!!!
thanks for the vid, i love the way you explain things so clearly!
Great clear explain ..gud teaching
I love your videos...You really make Chemistry easy and interesting
I love this guys content!
You are the reason I am actually learning chemistry
Wow..nice clear teaching
I wish I found you earlier... but you're definitely saving me on the final. Thank you!
Good bloke helps alot turned my life around i understand chemistry now thank u
Your videos have been fascinating, for the last mercury and calcium, calculation, wouldn't it be easier to use the formula 40.04/200.6 = 64.2/X? You get 321.21, which is close enough.
You are a legend
Hats off
Thanks for this perfect explanation !!
2nd Question:
Simply we can calculate by,
200.6g of Hg and 40.08g of Ca will have same no. of atoms.
They have ratio of 200.6/40.08 = 5.004
which means
64.2g of Ca will have 5 times of mercury to have same no. of atoms
Therefore, 64.2*5 = 321g of Hg
Your way of teaching is so nice.
great work and great explanation. May God guide you and bless you. Thank you
Thanks DeWitt for explaining things so patiently in terms even fifth graders would understand. All the best!
Thank you, you are amazing teacher.
You are awesome. Thank you for your precise hand movements.
This is great! Thank you!
thanks that was really helpful to get the concept of what mole really is. :)
Incredibly amazing and outstanding
Love the concept of "conversion factors"
This program is amazing you help us alot
I can jus say THANK you Tyler DeWitt :) love and respect for your work . From India
Simple and precise
Thanks, All of your vidoes really helped me
you are amazing 10x better than my teacher thanks for helping videos keep it up
you are the best, thank you so much
Thank You For Making Chemistry Fun For Me !
Amazing video, thanks aot
Keep it up Tyler thank you
keep going
amazing
Loved it!
All you need to do is look up how much that substance weights and then divide it by how much 1 mole is equal to for it’s short answer and divide the, and boom you have the answer. Keep up the great qork
Awesome
Life saving!
you are great
4:30 how do we know if we should put 16 grams at bottom of fraction line or top of fraction line ??
you are a life saver
Thanks Adam you have made my summer a lot better, but When they ask What is the mole ratio of salt to water? Ex. Mo(NO3)2 x 5H2O = 1: 5 yes/no or (CaSO4)2 x 2H2O = 2 : 2 yes/no
hay, can u make a video how u prepare a buffer solution with the Henderson-hasselbalch equation?
Hi Tyler, loving your videos, they are helping me a lot.
On this one however could you not simplify the maths (I'm English, we put an s on there!) by simply dividing 40.08 by 64.2 to find the ratio between the value we know and the value we want to know and then dividing 200.6 by that ratio? I know this may be more algebra than chemistry but it gave me the same answer in significantly less steps.
Keep up the great videos!
kijbvuuuhhjjbbbbnnnnnnjjjjjnjjjjjjjjjjjjjjnjjhhhjggggnnjhhhhhjhjšakkzzzzzzjjjmjjjnkjjklnmjjnnnmmkjnnkjkkpa£**&&&_&&&_^**_^^&&&&^^_*_^_
yes
About 9 years after this video was made this might just have saved my chem grades for tmrws test🙏🏻
For the first question I tried a different way...
16.00g/mol x mol/6.02x10^23 atoms which equals 2.66x10^-23 g/atom. Is this an acceptable way?
thanks way better than my teacher
🙌You the best💃💃
You're the best
really its helpful
Yo can u make video how we calculate this if its come out in mcq
you saved my life!
I did the second question differently and got the right answer and it seems a lot simpler:
64.2 / 40.08 = 1.6
1.6 * 200.6 =321.32...
me too
@@mrinfamous5037 same
And me, its just a molar ratio calculation. No need to involve the constant
I Only Want To Tell Three Words :THANKS,THANKS AND THANKS
Thank you
Thank you sooo much 👍😭💛
It's possible to make the last step a lot easier. Just leave the divided by and times *10^23 out. 9.64*200.6/6.02=321. Or just use the numbers given straight away.
200.6 (g/m Hg)/40.08 (g/m Ca) * 64.2 (grams of Ca) = 321.32 (the weight of an equal number of Hg atoms).
I think these two methods are faster and easier.
I thought that conversion factors also have infinite significant figures? I'm confused, for dimentional analysis, when do you count sig figs?
How does this man know exactly how to present exactly what we want
I think using moles in the 2nd question would be a lot easier instead of straight up atoms
+ tyler dewitt got a haircut 😂
best teacher
for the second question it might be preferable to solve it as a combined equation ie. m1/mm1 = m2/mm2. For me its more efficient doing it this way but its practically the same
But wouldnt the faster way of solving the second problem be from starting at 7:04 and finding the factor between 40.08 g of calcium and 64.2g of calcium; using that factor to multiply with the 200.6 g Mercury to get x grams of mercury?
I got confused by that part too . The most difficult part of this kind of question isn’t really the math itself, is the wording. As soon as I figure out what this question means we can just see how many moles are in 64 grams of calcium then we get 1.6. Then we multiply that with one mole of mercury then have the same result .... I don’t understand they way they do math here. It’s more complicated than how I was being taught back In my own country. But anyway the chemistry teacher is still awesome . Just the way he does the math confuses me LOL
Isn't it true that sig figs should not be applied when using conversion factors? So, the final answer shouldn't have 3 sig figs because of the atoms per mole value, but should instead stick with the initial number (1) offered for the number of atoms?
These videos helped me quite a bit. The issue I been running into in my chemistry class is illustrated in this example. Oxygen=16 grams. My periodic table says 15.99. Is it automatic to round this? seems inaccurate...
hey sam, yes it is, you do no thave to but it is done a lot of the time, you cal also choose to just round off at the end.
hope this helped.
I'm confused how I learned it is slightly different so can you explain to me why when you put the atomic number (bottom number of an element) you have a point like say copper is 29 but you have 29.something..?
Love from India 💌
I have a question why Avogadro's number is always 6.02x10 on power 23 in one mole of any substance 1 mole of water and one mole of an element ??
Does the Mass in single atom differ with the "amu" (atomic mass unit) ?
yes. the amu is mass of one mole and a mole contains 6.02 x10^23 atoms. in other words, the amu is the mass of 6.02 x10^23 atoms.
the question was asking for the mass of 1 atom, the 1 atom from 6.02 x10^23 atoms.
hope this helped ^-^
aylo ra Wasn't this molar mass? I mean it just asks for mass, isn't that 16?
aylo ra If thia was molar mass I could understand but I'm getting confused since it is only stated as "mass"
yes u are correct, the molar mass of oxygen is 16, means that the mass of 1 mole of oxygen is equal to 16.
but his question was asking for the mass of 1 atom of the 6.02 x10^23 atoms.
not the mass of 6.02 x10^23 atoms which is equal to 16 grams
aylo ra i wish I knew that before today's test 😂😂😂
Freakin awesome
gat damn lifesaver!
A question raised in my mind that
You told the 16.00u is molar mass of Oxygen molecule but it is the relative atomic mass of Oxygen and molar mass is 2×16u=32u that is 32u=32g=1mole=6.022×10²³.
So,if I'm right reply and if I'm wrong,reply
So, that I can clear my concept
Ur like a God to us
\i have put this into the calculator many times and have not come up with a negative exponent, please let me know what i have done wrong in my calculations thank you anna
try putting in brackets
sameeee
AHA! wait no its not about brackets what you do first is type into your calc 6.02 x 10 ^23 then press equals. then press the clear button and press 16 divided by ANS. THAT WAY THERE COMES A NEGATIVE EXPONENT!
me as well, so in that case i divided 16 by 6.02 only then because 10^23 is in the denominator, when u take it to the numerator the exponent becomes negative so 10^-23. hope u got it :"D
I see a lot of people in the comments talking about why isn't the Math done more simplified. Because he's teaching everyone how to do it. Meaning some people aren't as strong in Math and cannot see the shortcuts. So he's making videos for everyone. If you see it, then what's the problem? Have some consideration for those who need the extra Math help :)
Can someone please post all the formulas
Okay Nice
i ask you one question
1.Calculate the number of moles(n) containing 1.5x10 E22 a.m.u what's formula