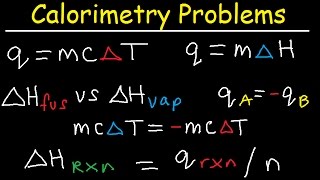
Food Calorimetry Lab: Calculations
HTML-код
- Опубликовано: 18 май 2012
- To see all my Chemistry videos, check out
socratic.org/chemistry
How many calories are in a food sample? We can find out by burning a potato chip, causing it to release energy. This will be absorbed by water in a calorimeter, so we will then calculate how much energy the water absorbed, using an equation for heat energy, specific heat, mass, and change in temperature (delta temperature). We will also calculate the Calories per gram and Calories per serving, and then calculate percent error to find out how far off out experimental numbers were from the real values.








You save my life every time I'm a dumb ass in chem.
@?
I honestly can relate to this so much, his videos help out so much
!
this guy is a blessing tbh. ive been helped by several videos. in this case my teacher just threw this equation at me and expected me to figure it out. thanks for the help guy lol.
dude... You are a life saver!! I had this same lab in class. I was trying to do the calculations and wasn't understanding anything. This really cleard things up. Thanks, you're the best!
Amazing...a teacher with the ability to teach!
Hey everyone, I'm here to help. If you have any questions or just want to learn more, click on the link in the description above. It'll take you to a page where you can ask me questions.
Tyler DeWitt suggest 2 reasons why the results may not be accurate. And how can we make it more accurate?
Tyler DeWitt are you still doing videos on chemistry?
Thanks Tyler, you're an angel
I love your videos I swear I learn more from watching ur videos than I do in class for 5 hours of chemistry
Not all heros wear capes
some wear lab coats
They wear pink shirts
Some wear pink v-neck t-shirts.
You taught me this in 4 minutes wow, my professor is just horrible. Thanks a lot!
professor? you learn this in grade 10
Broken Plane lol
@@XxSwagBeatboxerxX Believe it or not, people aren't interested in chemistry, but still have to take it in college due to a collateral requirement. So yes, professor.
@@DavidEaton98 didnt know that, thanks
@@XxSwagBeatboxerxX 10th grade? what school and what state are you in?
Hey Tyler! I am following your channel for a few weeks now and I got to say that you are rocking not only in America but in Israel too.
I am currently learning for an exam and struggling with 1400 pages textbook, thank you and a bunch of other great online teachers, the struggling decreases. thanks for that.
This is the greatest video ever and I think you just saved my chemistry grade. I've been trying to understand all these calculations for the past month and I finally get it and can now complete my lab conclusion. THANKYOUTHANKYOUTHANKYOU.
you videos are just amazing. its absolutely phenomenal how you make everything so easy to understand. very clear and to the point, every confusing. thank you so much.
Thank you so much Tyler you are truly amazing! You have helped me through Chemistry Biology and Physic please keep doing what your doing.
You have just taught me more than my teacher ever has, thank you.
I've been subbed for this reason. You're a great teacher! Thank you!
Almost 15 years later I now understand the Calorie and difference between “C” and “c” you’re great!
You're awesome! Thanks for being so detailed and having a great presentation.
THANK YOU!!! I was having such a hard time figuring out these equations. You were SUPER helpful!
This video saved my life on my chemistry lab thank you so much!!!!
This is an old video, but thank you so much for the video! It helped a lot, and I'm definitely feeling much more prepared for tomorrow's AP Chem Test because of this video :) You earned yourself another subscriber man!
very thorough, thanks so much! you saved my Chemistry Lab project!
you literally just saved my grade on this formal lab report, thank you so much
thank you for saving my chemistry grades
I love this Dr. Tyler!!!! I'm big fan of yours
Very organized and clearly explained !
I so wish you were my Chem teacher you explain it so clear and simple. I do not get the same understanding in class. Thank you so much!
You're my chem saviour. But can I ask? How come I get 9.5%? 210-190=20/210=2/21 or 0.0952 x 100% = 9.5% ??
Exactly my point
Thank you so much this video saved me from a TON of stress!!!
excellent video. Very clean and precise.
This helped with my lab luv the way you break down the information because I wasn't able to find that in text book and I wasn't able to get in on a session on online tutoring. So thisjust made it easy for me. Thank you! :)
Had to watch this for science, it's going to be really helpful in class :)
hey Tyler know anything about measuring energy - specific heat?
Perfect! Lot of thanks to you. It very helps my project! Again thank you very much! 👍
Thanks m8 helped me a ton in my science project!
You are an excellent teacher
Your videos are the best, man.
Skip to 4:18 mark to see beginning of the explanation/equation for how to find the heat released by the snack in food Cal.
Tyler I want to thank you for your efforts. I am a HS science teacher & I augment my instruction by using videos from people like you. When I get a transfer student that has gaps in their education, I point them to folks here like you. When I am absent, I often use videos for the sub to show. I am also moving to a project based learning environment & I often have students watch videos as HW and then we get together briefly to discuss the info, and then spend our time on labs!
Very helpful video. Will link for my students.
Thank you sm, I understand everything now, I have a lab tomorrow on this exact concept and I was able to plan out my whole lab. Thank you sm, may god bless you
great explanation, thank you
amazing I had an Energy online quiz and I only had 4 mins left and I did it correct. If 200 g of water is heated from 5 C to 25 C, then what is the heat added in kilocalories (same as food calories)? 200*20= 4000 = 4000/1000 = 4 kcal. Thanks Tyler
This is so helpful. thank you so much
Thank you I was stuck this really helped teachers don't explain
I'm confused because I'm pretty sure my teacher told us that for finding the number of Calories per gram we needed to divide the amount of Calories by the CHANGE in mass of the chip after it was burned rather than the initial mass of the chip as it was done in this vid... this way makes more sense so i'm not sure if my teacher misspoke but when i do it this way my percent error was so high
I wish this guy was my chemistry teacher!
What I was taught about percent error is measured value - accepted value OVER accepted value (that answer) times 100
Thanks for helping me with my study guide
oh my god you helped me so much!
Isn't the percent error: 9.5% and not 11%? (210-190)/210?
that's what I got too
How can you lower your percent error (what modifications can you make to the lab) so it can get closer to 0%
this video saved me in my chemistry class
What bugs me is how you can ever warm up 150g water by 65 degrees celcius by burning a single potato chip. Id have enough trouble getting it to burn at all. If youd start at a moderate temp of 35 deg, that water would be boiling! What kind of crisps are you using? Made of gun powder?
G.O.A.T = GREATEST teacher OF ALL TIME
That was very clear! Thank you!
how come you dont use 4.184 as the specific heat capacity for water?
Jonathan Alban It's just different units. The way 12 inches equals 1 foot. 4.184 Joules is equal to 1 Calorie. So water has a specific heat capacity of 1 cal/g•ºC, or 4.184 J/g•ºC.
+Tyler DeWitt but when you use 4.184 Joules, you get a totally different answer
+Tyler DeWitt 4.184 Joules equals 1 Calorie, not 1 calorie. You then converted something that was already in Calories to Calories, because you put that 1 Joule equals 1 calorie.
+Fresh StopMotionReviews No, 4.184 KILOjoules equals 1 Calorie (uppercase). 4.184 Joules equals one calorie (lowercase). I don't fully understand what you're saying in your comment, but my math is right here.
Does heating the potato chip Add energy to it or is the added heat Only converting the chemical energy in the chip to its own heat energy?
Excellent!
Very.... Nice.. Thank you sir....
post more dood realy helpful for my science exams 👨❤️👨
I'm eating hot cheeto puffs. just figured out each puff has 12 frikin Cals in it. Imma go burn it w/ fire
Can't we just plug in the specific heat of water for (c) instead of writing down (1cal/g xCelsius?
You. Are. Amazing.
W teacher as always
how do I solve for energy content if only one temperature is given?
ex: my test problem says
"A 1.0 g sample of food reacts with oxygen in a calorimeter containing 500 g of water. The temperature rises 10°C. What is the energy content of the food?" do i subtract the given temp from 100°C or just multiply the given temp?
I think the difference of temperature is already given cause u ve said " rises 10°C
means it was in a temperature and it rises 10°C to be in a another temperature
I think just multiply
god bless your teachings
Thank you so much
the heat capacity of water for c is 4.19 J/
How do you get something to burn underwater
Thank You
GRANDE DESDE LATINOAMERICA!!!! GRACIAS
How would I solve this, I am so confused...Using dodecane, the calorimeter constant was found to be 28.9 kJ/°C, calculate the amount of kilocalories (Cal) per gram given off by a unknown sample if burning 0.5 g caused the temperature of the calorimeter to rise from and initial temperature of 25.0 °C to 30.5 °C. (Reminder: food labels use calories (Cal); 1 Cal = 1000 cal; 1 cal = 4.184 J)
Ohh mg god thank you so much for this video.... I was crying before this explanation......
Why not do initial temperature minus the final temperature? That is what i ma doing for my chemistry lab.
This man is true hero!
I'm pretty sure you are actually supposed to divide the Calories by the change in mass, not the original mass. You do this so that you can actually find how much energy was released and not extra mass
what application are you using to shoot diz videos?
Please , this is so hard can you like explain it more in the video "Food Calorimetry Lab:Explanation"
Thanks, this will be helpful if you did. :)
wouldn't we use the mass of the potato chip? i did this with a nut in school and we measured the mass before and after the burning..
how many kcal of heat energy did the potato chip release when burned?
Would you tell me how many grams of potato chips can I burn to produce heat?
187 calories bro!(elps: 8:50) you missed the equation :) anyway your videos are awesome!
He used significant figures
how would you convert it to Joules?
how did you get 11% for the percent error? i got 9.5% when i calculated for percent error?
Thanks
Thanks!
ur awesome, thanks
You didnt had to confuse on taking simple method on Proportion and ratio of calories to kilocalories .
1.75 g is the mass of potato chips burnt to raise the temperature of water, right? Not the initial mass of potato chips, right?
You have just saved me. You are a God.
lets not get carried away now
why is my percent of error 9.5% i inputed |210 - 190|/210 x 100% is there some mistake I've done?
Hi, to figure out the Calories per gram, was 1.75g the initial mass?
I'm literally looking for this comment but no one has replied for 7 years...
But i want to know how to figure out how many calories are in 1 whole bag of chips..
Hi I just have a question...when I do the math out to calculate percent error I am getting 9.5%. Can someone explain how the percent error stated in the video is 11%??
I am also getting 9.5%
amazing
37 people got hit with too much knowledge and missed the like button
tysm
Can calirimetry or a similar process be used for liquids? Say if i wanted to find out how much energy is in different sodas??
Yes, but you'd have to evaporate them first, because water can't burn. So like for a soda, you'd evaporate it, and then the sugary goo that's left over at the bottom of the glass, you'd burn that and do calorimetry.
Tyler DeWitt so I could then afterwards do an experiment to test the amount of energy each releases? I'm trying to come up with an idea for a chemistry investigation and really struggling
Yes.
How did you get 1.75g patato chip?????
The percent error is 9.5%
i got 9.5% error... but if I took absolute value and divided it by measured value, I get around 11% error