Constitutional Isomers
HTML-код
- Опубликовано: 8 фев 2025
- This organic chemistry video tutorial provides a basic introduction into constitutional isomers. It explains how to draw the constitutional isomers of butane, pentane, hexane, heptane, and octane.
Organic Chemistry - Basic Introduction: • Organic Chemistry - Ba...
Organic Chemistry - Video Lessons:
www.video-tuto...
______________________________
Acids and Bases - Basic Intro:
• Acids and Bases - Basi...
Lewis Acids and Bases:
• Lewis Acids and Bases
Nucleophiles and Electrophiles:
• Nucleophiles and Elect...
Hydrocarbons:
• Hydrocarbons - Aliphat...
_______________________________
IUPAC Nomenclature of Alkanes:
• IUPAC Nomenclature of ...
Naming Cycloalkanes:
• Naming Cycloalkanes Wi...
Naming Bicyclic Compounds:
• Naming Bicyclic Compounds
Naming Ethers:
• Naming Ethers - IUPAC ...
Naming Alcohols:
• Naming Alcohols - IUPA...
Naming Alkyl Halides:
• Naming Alkyl Halides -...
________________________________
Naming Amines:
• Naming Amines - IUPAC ...
Van Der Waal Forces:
• Van Der Waals Forces
Boiling Point of Organic Compounds:
• Boiling Point of Organ...
Organic Chemistry PDF Worksheets:
www.video-tuto...
Organic Chemistry Exam 1 Playlist:
bit.ly/3kJnNXU
Full-Length Videos and Worksheets:
/ collections
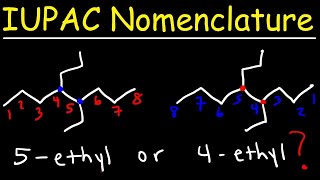







IUPAC Nomenclature - Full 42 Minute Video: bit.ly/3GURyOl
Organic Chemistry - Video Lessons: www.video-tutor.net/organic-chemistry.html
All hail organic chemistry tutor 🎯
sorry to be off topic but does someone know a method to log back into an instagram account??
I stupidly lost the password. I would love any help you can offer me.
@Misael Justin Thanks for your reply. I found the site thru google and Im trying it out atm.
Takes a while so I will get back to you later with my results.
@Misael Justin it did the trick and I actually got access to my account again. Im so happy:D
Thank you so much you saved my ass :D
@Remy Brandon no problem xD
@@remybrandon6522 bot
My teacher too bored to teach online so you are a blessing
I learnt in this 13 min video more than the 5 hours session I took at school, Thank u very much
Your dedication to your students is truly admirable and I'm grateful for all the extra time and effort you put in to help us succeed.
I find you the best and I search your videos in every topic I don’t understand in organic chemistry
You are a blessing.
Super explanation on organic chemistry
Wow!! You deserve honor 🙏👏👏 Thank you so much sir!
At this point why do I even pay for my college education when I can watch this for free and learn more🥴🥴
haha for the college degree
btw this is in college curriculum? im in india, and this like early days of high school
@@disciplinedevsomething like isomers is in high school but high school barely touches on organic chemistry we only go over a chapter or 2 of Ochem in the us you don’t really need organic chem until college and even then it’s only necessary for pre pharmacy and premeds and chem majors
If this is all and less being taught in a college, what college even is this
It's very useful. Thank you🌻
this is the best video ever
if you were my prof, chemistry would be so much better
btw to find the number of isomers for the first 7 alkane use C^(n-4) +1 , when n is the number of carbon atoms
????
It's is so worthwhile
Thank you for what you do.
Hey this is Orgo, pay attention this will 100% be on the midterm.
For the C4H8 @ 10:45 , shouldnt there be another isomer = 2-methylpropene?
@4:59 if you put the ethyl group on carbon 2 wouldnt that be another isomer?
Yes. I was thinking the same
same
Are you ralking about the bottom right one? If yes, then i dont think so... Cuz if u name it it will stil be 3methylhexane, which is the same as the top right one.
Amazing lecture
Thank you so much (Feb 5, 2024)
Thank you so much !
thank you for your help!
is there a formula to compute how many isomers a compound has?
ruclips.net/video/YRPbxMR3_Ug/видео.html
is 2-ethyl pentane possible?
Hi from Stanford student here.
Should it be C6H12 not C6H14 since C6H14 is hexane and C6H12 is the pentane in your video about "HYDROCARBONS"
1:23 what is the IUPAC name of this? Neopentane isn't it
Pentane on the upper left and 2-methylbutane on the right
@@oscarmantecon5354 Sorry, i meant bottom left.
@@Antweezy 2,2-dimethyl pentane adheres to IUPAC, but it might have a more colloquial name that I'm not familiar with.
no dumbass. thats a trivial name
Thanks a lot🙏🙏
4:42 why isn't 2-ethylpentane an isomer as well?
Putting the ethyl on carbon 2 would make the carbon chain shorter than 5. It’s basically no longer pentane because the continuous carbon chain would be 4 carbons instead of 5.
Do we only add methyl groups
thanks for this. But we could also have 2-ethylpentane?
the IUPAC name of 2-ethylpentane is 3-methylhexane
Why can't you put the ethyl on another part of the molecule instead of just the middle? C7H16
in 2:24 is it really 2-methylpentane or it should be 2-dimethylhexane, because C6H14 is known as hexane??... Just asking po hehe
Sir is there any formula that we can use to find out the number of isomers an alkane has
Thanks 🌷🌷
Thank you (Fri 18 Dec 2020, 3:02)
Is there a formula for the number of constitutional isomer
2,3 Dimethyl Pentane . How many isomers does it has ??
how would you figure out how many constitutional isomers for C4H9F?
Is there any formula to find the number of isomers ?
Nope
Yes there is! Go search "Komali mam isomerism trick" it helped me
@@Joeythegoats thank you😀😀
In C7 H16, how about this? 3-ethyl-2-methyl-butane. Wouldn't that be another isomer?
Can't we place 2 methyl groups on the end carbon
In C6H14 we can't add the isomer 2,2,2-trimethyl propane?
If it existed, you could.
king
Why is neo-pentane not a constitutional isomer of pentane?
Can someone recommend a book for studying the basics of Organic chemistry?
Nice bro
how do you know which isomer is the most stable or least stable?
did you find an answer?
Does the amount of hydrogens have any say in the different nomenclature structures there will be?? For example C8H18 when you said there was 18 different structures?? Is that consistent with all constitutional isomers or that example only? Thanks :)
That example only I think. It's not a linear scale. Ie. If you had C12H26, there would be more than 26 isomers. I think.
For C7H16 there were 8 isomers.
No relation dude
I think this will make me crazy
In regards to the C4H8 the cos and trans examples the hydrogens seem to had more than 4 carbons. Do we not count the hydrogen added to the structure ?
Maryann Chiang that’s what I’m wondering as well..?
why cant u add propyl to the group
why only ethyl and methyl
it does get pretty confusing and i was wondering the same thing. but it became clear after his C8H18 example. this is because if you attached a propyl it would form a longer carbon chain and be identical to the other isomers you already counted. this would sometimes happen if certain additions of ethyls.
Or any relation between the no of hydrogens and no of isomers . Can u Pls ans sir
There is no such relation bro
I don't understand it?
I would suggest you make your videos a little longer, thanks.
at 2:20 the structure on the right type, its still hexane ( 6Carbon ) isn't? how pentane?
Hexane means there are 6 carbon atoms all in a row, that one has 5 in a row so it's parent carbon chain is pentane.
Ryan W thank you .. it’s now clear
I want to see you