Dalton's Law and Partial Pressures
HTML-код
- Опубликовано: 11 дек 2024
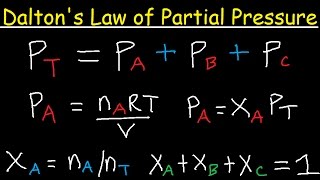
- We know about the ideal gas laws, like Boyle's and Charles's and so forth. Now let's look at one law that involves mixtures of gases. This is called Dalton's Law, and to understand it, we have to learn about the concept of partial pressures, so let's take a look!
Watch the whole General Chemistry playlist: bit.ly/ProfDave...
Study for the AP Chemistry exam with me: bit.ly/ProfDav...
Organic Chemistry Tutorials: bit.ly/ProfDave...
Biochemistry Tutorials: bit.ly/ProfDave...
Biology Tutorials: bit.ly/ProfDaveBio
Classical Physics Tutorials: bit.ly/ProfDave...
Modern Physics Tutorials: bit.ly/ProfDave...
Mathematics Tutorials: bit.ly/ProfDave...
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: amzn.to/2HtNpVH
Bookshop: bit.ly/39cKADM
Barnes and Noble: bit.ly/3pUjmrn
Book Depository: bit.ly/3aOVDlT








Where are you?? You made this five years ago but five years later it's helping me
As an A&P Student who is struggling with this subject, I have to say, thank you!!! This is the first time calculating a partial pressure has made sense to me!
EVERYONE who sees and benefits from this man should donate to his patreon. His back must hurt for carrying all our grades!
Man, your intros are my favourite.
@@KurosakaBlack and Chinese
@@Dazaioak ???????????
I’ma be honest 💀 this is my 2nd time taking Chem 1212L & I used to skip your videos but now that I watch them, they’re VERY helpful. You explain it SO easily bro, this chem shit is easy after watching your videos, thank you!!
Yo that was amazing, thanks for helping,you got a subscriber 🙋🙋🙋🙋
Your illustration is very CLEAR !
It is just what I needed for my lab report ! Thank you
Thank you again for another great and informative video Professor Dave!
Thank you, surprisingly I really struggled with the subject when it came to answering questions.
Honestly you are very detail and simplify everything 🔥🔥🔥tnx Prof
Danke sehr gut und praktisch, unserer Professor hat diese link als ein schöne Erklärung von Dalton's law erwähnt
you are a life saver, your channel helps heaps
Great explanation, but the units bother me. When will chemists learn to use SI units..?
Great video sir. THank you!
I genuinly think binge watching his videos would help me more than going to my college lectures
Go to your lectures buddy
Great channel and video. Subscribed.
Thank you sir
That intro just got you a sub lol glad I found you 🤣
Thank you
Good explanation
Thank you professor Dave
I always understand you so easily. Moreover my bro is Dave
As usually as good always prof D
tq sir, wow what a explanation sir👌
Really nice one
Man I follow all of you are video it was great
thank you so much ure saving my life
Great job, thank you for your precious help
Heard Torr unit for first time. 😮
How does sparging work, when the partial pressure of each dissolved gas is independent of the partial pressure of the sparging gas?
Nice
Thanks sir please make a video on numerical part please
If you want to get numericals on dalton's law of partial pressure
ruclips.net/video/N-XpFlD_lJk/видео.html
here before my science test in an hour
Sir can you make flow diagram on Daltons law of partial pressure
Please make a video oN calculus module one
I have an entire calculus playlist, bud.
He divided the percentages by hundred because they equal to 1atm.(note for me)
All the pressure are in gauge or absolute ?
You have to use absolute pressure in gas laws.
Sir can you plz make a video about ionic equilibrium
no u
@@38Fanda bro thought he was funny 💀
What's the answer?
Do they still use torr or mm/Hg? I had hoped everyone would use pascals by now.
Torr is pretty standard.
@@ProfessorDaveExplains How does sparging work, when the partial pressure of each dissolved gas is independent of the partial pressure of the sparging gas?
please explain more with examples
ruclips.net/video/N-XpFlD_lJk/видео.html
Go and watch lecture videos
With numericals and example MCQS
You should show how to work the solution not only the results
The calculation is very clearly shown.
@@ProfessorDaveExplains Jose just wanted to make a comment thats all
👍
3rd comment...
no matter how much people explain to me i still dont get this what do you mean partial pressure and 1 atom i dont know this i dont get it watched like 5 videos already
👋🏼👋🏼👋🏼👋🏼👋🏼👋🏼👋🏼
I like taocs
Me too
Bruh
He looks like Ranbir Kapoor😂
😂 certainly
Konse angle se bahen
👍👍👍
Arre Gjb
Kitne number aaye?
Cool
Second comment
chemistry jesus/ science jesus
❣❣❣
:-)
bro I have an exam in 1 hour on this! Clutch af
Did you kick butt or get your butt kicked? Was it clutch af enough?
Nice