Quick Revision - pH titration curves & indicators
HTML-код
- Опубликовано: 23 май 2019
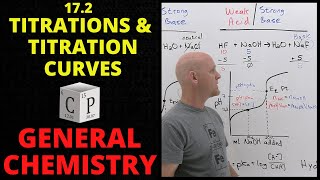
- Video looks at the key zones of a pH titration curve then goes into more detail for the different acid-base combinations.
pH at equivalence and buffer action also covered.
Video finishes by looking at how to select the correct indicator








This man changed my life
In a good way I hope!
Ur explanation is on point ..thanks a lot for this video...subscribed because of this video...
absolute icon - thank you so much sir!
You're welcome!
an absolute king
Machemguy is a hero
I'll take that
thank you so much
Legendddddd!!!!!!
do we need to know about buffer regions for ocr a?
Could always be a suggest question. I tell my students about it
in the specification, it talks about the indicator as a weak acid in terms of equilibrium.. so how does it work?
Indicators are weak acids (HIn)
So partially dissociate
HIn H+ + In-
Each side of equilibrium has a different colour
You can apply le Chatelier’s principle to explain effect of addition of acid/base and hence the colour it will go
@@MaChemGuy great, thanks a lot. i appreciate your work
5:25 when all the acid has been neutralised, where are the ethanoate ions coming from? Are the ethanoate ions that form the buffer coming from the dissociation of ethanoic acid or from the dissociation of sodium ethanoate?
p.s thanks so much for such helpful videos
They’re a product of the reaction, so yes, from the complete dissociation of the salt
@@MaChemGuy thank you so much for your quick reply! I think I understand now: acid and base forms salt + water, then this salt formed dissociates to form ethanoate ions.
@@daisylawrence7447 You got it! 👌
In 1 at 0:25 does the pH not increase because the basic solution is added?
Yes. But only very slightly, because you are adding base slowly, and the acid is still in excess. The graph line is not flat, but goes up only as a very shallow incline at this point.
How would you draw a pH curve ?
Nice video, but what about the indicators? 😳
Did you watch till the end?
Oops, an ad cut it off and I thought it was the end of the video. My bad, thank you for all the great content 😳👍🏻