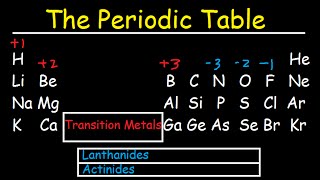
A Tour of the Periodic Table
HTML-код
- Опубликовано: 16 окт 2010
- Mr. Andersen describes the major groups on the periodic table.
Intro Music Atribution
Title: I4dsong_loop_main.wav
Artist: CosmicD
Link to sound: www.freesound.org/people/Cosmi...
Creative Commons Atribution License








This man is amazing. I wish RUclips had been around during my college years...would have saved me many tears.
very true
britishTRIGGERED chick 9th as in high school? Cause me toooo
I'm left with a week to start my final exam,I need more information on chemistry...I don't know where to start
Same thought!
Let me tell you, It doesn’t change a fucking thing about college. Still sucks ass
2021.. after a decade he is still the best chem teacher. Helping students like me across the globe. I am preparing for a hardass exam and this is the most helpful channel I have come across. Incredible. Better than any paid course or even Uni professors.
This channel should be trending now because of Covid and online classes boom.
I laughed at that disappearing hand joke for 5 minutes straight lol
lol
Disappearing hennd
SAME AHAHAHAHHAHAHAHAHAHAA
I was waiting for you to start with the tour when you were talking about the book but it was definitely worth the wait! Thank You! =D
Fascinating! I tutor an 8th grader in Science, and this is perfect for her. Thank you for the tip on the alkali metals in water videos, so awesome! She'll love that.
N5 Chemistry in Scotland numbers the groups from 0 to 7 missing out the transition metals. Also, not all gallium is radioactive.
Thanks. Easy to understand explanation of the Table. Love the highlighter pen and photos.
Thank you! You're videos are helping me study for my nursing entrance exam! Much appreciated.
Thank you Mr. Andersen for this youtube lesson! :)
The Disappearing Spoon is one of the best books I have ever read, in my opinion. I love it!
I just finished reading "The Disapperaing Spoon." It is an excellent book. I am currently reading "The Violinist's Thumb." Sam Kean and Mr. Anderson should create textbooks together.
@ 0:52 I thought he was making a smiley face
same wtf
me too
same lol
me tooooooo
You need to update this video to include the several elements near the end that have been discovered since you made this. I like the fact that you put Lutetium and Lawrencium in their correct places among the transition metals, however these two series are now called the Lanthanoids and Actinoids. Polonium is sometimes considered to be a post-transition metal, but I like it as a metalloid because it completes the stair-step pattern :)
Cool! this helps me remember all the things I forgot again!
Gallium is NOT radioactive! In more detail: the naturally occurring gallium is composed of two isotopes, Ga-69 and Ga-71 - and both are stable.
Correct. Also far less toxic than mercury.
Gianmario Scott
my thoughts exactly! I'm actually watching this as a required assignment for chemistry class and I have gallium at home. Curious how this slipped by my teacher...
Darn it I was just about to say that.
My thoughts!
this was a lifesaver, thank you so much!
CAME TO STUDY, LEFT SUBSCRIBED
Thank you sooooo much I had a hard time understanding the periodic table. Will watch again and take notes before class starts!
This was very helpful, thank you for that :)
love your work
really helps a lot
BIG THX
He seriously messed up the metals and non metals separation part
HI 4 years
@@br3ys3n16 lol.... but wha- like- i have a test today.... did he do something wrong?
@@zuzuderose1246 what grade are u in?
How
Damn it’s been 4 years since I commented this, and I don’t even remember doing it
Roses are red, violets are blue I came here to study, so did you
No I came here for the meme
I came to read your comment
Me too
I actually didn't I was just interested in it.
"Galium is highly radioactive" are you sure about that
Thank you very much Bozeman Science. Your videos are very helpful.
Awesome! Thanks a million !
We LOVE Mr. Andersen!!!!!!
Excellent!
Mr. Anderson, what video software are you using to create this video? btw love the video!
Thanks.Your keeping our science straight. Thanks
just ordered the book! so excited to read it!
Your so smart and kept my interest throughout the whole video!! You got my subscription thank you this helped for my final so much!!!
I always hated chemistry... It's intimidating like hell
Love his so informative - I learnt to much .
I have seen a number of videos handling gallium, so I think it is not normally radioactive
you are so amazing! thanks a lot!! from korea
Excellent Excellent Excellent explanation....please keep it up and and upload similar stuff...
This is awesome!! Very helpful... Thank you :)
thanks for this. helped me heaps
very well explained thank you
thank you very much!
excellent video!
This was very helpful thinks mr:Andersen
Thanks for making the table more regular to me by explaining that the last 2 rows should be a lot longer :D
Also why isn't [Al] a metalloid?
And finally are the 2 loose rows transition metals as well?
+sinekonata
Hello!
1.Aluminum(Al) is a metal since it's shiny, ductile and malleable.Also, it loses valence electrons(just like any metal), and is a solid at room temperature.
2.The last rows are called Lanthanides (1st row down) and Actinides (2nd row down).They are called "inner transitional metals".One reason they are separated from the rest is because they have an f orbital.To make the periodic table simpler and more organized ,they placed the elements in a way that the s,p,d and f orbitals elements are beside each other.
Wish that helped ;)
Nice work, good communication skills.
great vid...clean and articulate!!
Gallium isn't radioactive. It has unstable isotopes, but all elements do. Some are naturally occurring, and others are produced synthetically (proton, neutron, or heavy ion capture). There are Plutonium-Gallium alloys, which are used in reactors though.
Thank you for helping me with my Chem assignment!
Thanks for your time to make such good education vedio. I use it teacher my son.
what video maker do you use! awesome vid btw! helped me a lot ... way better than most of periodic table vids ive watched =)
Thanks will be taking chemistry for the first time I never took this subject in high school so this is a need introduction to the periodic table.
Very helpful! Thanks.
Świetny film
Thx! Very good!
Thank you
Thank you for doing this! I homeschool my kids and this helps out so much!
Nicoya_Beauty why?
You shouldn't they need a social life.
Sometimes if parents are smart or their kids get bullied they just homeschool them:) And they do make Homeschool co-ops, they're like one day a week private schools so that they DO socialize. And I know a homeschooler, and they are probably the smartest person I know... so good on you Nicoya!
Thanks. Try list-twist for the elements
Great video I like it you make more sense than my teacher
amazing Sir
you teach very well
very good thankx
good explaination about elements n their place in peroidic table
on the transtion metal of group 2B is like that Zn are similar with group 1A
i liked the video just by seeing the thumbnail,later saw the video
THANK YOU!!!
Dang this helped me!
i love it
Thank you!! :)
Thanks!
8 groups if you're talking about the specific groups like the transition metals, alkali metals etc. There are 18 'groups' or families, as in the columns. I guess he used them interchangeably.
I'm not learning this in chemistry yet but I like to learn more about what chemistry is about.
excellent
Science Homework is Lit
very nice tutor!!!
Thankyou... niga adipoly aanettaa
Also, I believe that the noble gasses all have a filled outer electron shell, not 8 valence electrons, for helium has only a couple of electrons up to 4 and radon has a large outer shell capable of 18 or more, I would have to look it up.
Electron configurations please make a video!
very good
very nice video thnks fr the help
I came here to do late summer homework bc my mom is going to beat my ass if I fail and I have an 18 in science
felt that
Using this for homework
THIS VID IS MORE USEFULL THAN MY TEACHER VID
Can u explain what u mean by valance -and explain how lost and gain electrons
well that ended unexpectedly
Good one.
hiiii
can you tell me please what ddi you use to make this video what is that tool you use to appear on screen like a black board???
Thanks
And just like that we’ve gone across the whole universe
the number of valence electrons increases going from left to right so the noble gases have eight because the halogens before them have 7
I believe that Gallium is not radioactive, but rather the Isotope Gallium 67 is. Regular metallic Gallium is considered non-toxic to the body, but Gallium salts can cause renal trouble.
Every atom has valence electrons, unless it has no electrons at all (such as ionized hydrogen). The definition of a valence electron is simply an electron that is capable of forming a covalent bond. Though the noble gases are not apt to do so under normal conditions, they can form bonds if their electrons are excited to a high enough energy level. Xenon is particularly easy to form bonds with in respect to the others.
very nice sir
thanks
2:35 wait so 87 belongs on that group or not?
It does. Francium is the most reactive metal
that is quite interesting actually. my understanding of 'inert' in a chemistry context means an inability to chemically react, i.e. form covalent/ionic bonds with another element. When you ionise elements it doesnt mean they are chemically active, nor does the fact they can form excimers.
No isolated compounds have been made, but certain ions and excimers have been detected. Therefore, they're not completely inert.
can you do one about metals and non-metals
What software do you use to make these?
Hey guy answer my question if helium has two electron in its last shell so its balance but it should be on 2nd group 1 shell 1 period is true but how its noble
I’m here from summer homeworrkkkk....
:D
gallium is completely safe and readily available to the public. not radioactive whatsoever...
+Tim Miller Some isotopes of gallium are radioactive but the most common are stable. So go on play with it. :)
But isn't valenselectrons just a name for the electrons located in the outer shell? Even if there are eight electrons in the outer shell, it's still eight valenselectrons, since the outer shell is also called the valens shell, right? I may be wrong though, haven't really read into this enough yet.
ty