Alkenes and Alkynes - Naming + Properties
HTML-код
- Опубликовано: 27 июл 2024
- Alkenes are hydrocarbons with DOUBLE bonds. Alkynes are hydrocarbons with TRIPLE bonds.
They are named exactly the same as alkanes, but with ENE and YNE endings, and you might have to give a number to show where the double/triple bond startings in the chain.
Check me out: www.chemistnate.com
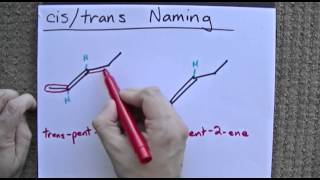







3 Hours in school : 8 minutes in youtube
Noureddin AbuMutawe absolutely correct!
You are very KENE in Chemistry and very KYNE for teaching us.
Thank You!
I'm pretty sure I'm not the only one who started to love chemistry after watching these videos :p
Nur Shahira no
You are
I’m not going to lie I was playing PUBG when I should have been learning (only because I have a substitute right now) and after watching just four minutes of this video I started to understand the subject because you got to the point right away, thank you so much!!!
Your answer is wrong at 5:10. It should be oct-6-en-2-yne. Priority goes -OH, triple bond, double bond, halogens, single bond.
Npe...if they both are in main chain double bond has more priority
Chemist Nate, you are AWESOME! I aspire to become as smart and talented as you are at chemistry one day. You're the reason why I get good marks this year.
Yushi Liang b
Great summative video, includes lots of relevant information ^^
Also, I finally worked out who you sound like... you sound like Ryan Reynolds haha
Hey man! thanks for making these videos concise and to the point!
Thanks so much! You keep making and I"ll keep watching, and learning.
really helpful video. It makes a difficult subject seem so easy.
Your videos are soooo helpful, can u make more based on organic chemistry?
Thank you so much Master NATE!
"You know? ..Its a party. All day. Every day. Good luck."
It was a great explanation. keep on doing so.
Thanks for doing this!
Thanks for sharing it's very interesting
Great video
hii can u plz explain me how have u put the naming to exactly opp position???...... like first u started it from right hand side but when u changed to a diffrent sheet u started naming the name from left hand side even though they were triple and double bond together...... u still have consider double bond even though triple bond was there also ...!! was it because it is lower bond than triple that's why hve u consider it or with a diffrent concept plz do ellaborate it >>
Thank you so much! :D
Super helpful!!
Do you have any vids on sub shells, orbitals , and electron configuration etc
Sir plz make video on bicyclo compound's
thank you!
my teacher use iso- neo- as prefixes in alkyl groups. I'm so damn confused where and how to use them. i doubt its not used in IUPAC naming anymore but i see words like neopenty- , isobutyl- all the time in my test papers. I'm stuck even after watching Nate's video.
Soo very helpful 😀
fantastic demonatration thanxxx
Great explanation! But if we have both double and a triple bond, we label the double with the smallest number? Not the triple?
It would be whichever one COULD get the lowest number. And if it's the same for both (i.e. the molecule has a double bond at one end and a triple at the other) then the double bond gets the lower number. It's an arbitrary rule but here it is. chemistry.stackexchange.com/questions/28202/why-are-enynes-named-as-en-yne-and-not-yn-ene
U r great
it's great
Why dimethyl please explain I dont understand why you put that there.
Because it has 2 methyl that is why "di" is included. Di means two
omg thx bae for this vid
Does anyone know why he gave ene the lowest no and not the yne at 4:10
He gave ENE because it is only said double bond . See in the video there is only 2 Lines .
In " Hex-2-yne " It ended with YNE because there are three lines . For ALK-YNE
No sir the highest of boiling point is the alkane because of the presence of 4 sigma bonds and the lowest is the alkyne it have 2 sigma bonds and 2 pi bonds
trust me on that for now................lmao! that was hilarious
I did the IUPAC version on my chem test but it was marked wrong. And the correct answer was the outdated version ;(((( thats why im looking on RUclips to see why I was wrong.
I’m taking organic chemistry this year and have been really stressed about it. I follow RUclips since it’s easier for me to understand, but when I do it on the tests, it’s marked wronggn. Mostly because I’m using the IUPAC version and not theirs...
too complicated whenever you go deep in chemistry
iupac,a group of british ppl who control how to name it hahahahahahahahaha
alkyne takes priority in numbering not alkene..
In the end it doesn't matter. They are equal
alkene takes first priority than alkyne
Panee B alkene takes first priority than alkyne
In our discussion, alkyne is the priority rather than alkene. It's so confusing
Thanks for sharing it's very interesting