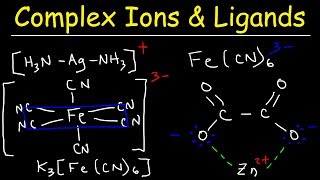
Coordination Ions Properties | CHEM102
HTML-код
- Опубликовано: 18 сен 2024
- strong weak field ligand, wavelengths, crystal field orbital drawings for octahedral (6 coordination number).
PS: 2 PARAMAGNETIC ions can be compared by:
1. High spin (weak field ligand) - (e- spend time in higher orbitals, as a result, more unpaired e)
2. Low spin (strong field ligand)
Diamagnetic will just be diamagnetic compared against paramagnetic