Amino Acid Stereochemistry R and S vs D and L Configuration
HTML-код
- Опубликовано: 7 фев 2025
- leah4sci.com/a... presents: Amino Acid Stereochemistry finding R and S or D and L on Fischer Projections and linear molecules.
📺Watch Next: Amino Acid Stereochemistry R&S vs D&L • Amino Acid Stereochemi...
Is your MCAT just around the corner? Grab a free copy of my ebook "MCAT Exam Strategy - A 6 Week Guide To Crushing The MCAT" at leah4sci.com/M...
This is video 4 in the MCAT amino acids tutorial video series. How to determine if amino acids are R/S or D/L from linear and Fischer forms and how to quickly convert between them.
Referenced in this video:
Chirality tutorial series: leah4sci.com/c...
Catch this entire video series along with my amino acid cheat sheet, tutorials and practice quiz on my website:
leah4sci.com/a...
Looking for guidance on how to tailor your MCAT Self-Study journey to fit your unique background, experience, and personal goals without feeling alone in the process?
That’s what my new MCAT program is all about, join me here:
leah4sci.com/s...
Need more help? I offer private online MCAT tutoring. Details leah4sci.com/m...
Have questions? Leave a comment below this video or hit me up on social media:
Let’s connect:▸Instagram: / leah4sci
▸Facebook: / leah4sci
▸Twitter: / leah4sci
▸Pinterest: / leah4sci








This video should be obligatory viewing in any organic chemistry/biochemistry class that teaches L vs D orientation. Amazing
Haha glad it helped
Definitely the best
Four years later and this video is still helping students! Thank you for helping me with Fischer projections of amino acids, something I was really struggling with!
You're very welcome, glad it helped!
Paused my biochem reading for lecture to learn a bit more about L & D since I never saw this in O-chem. Appreciate the thorough lesson!
So glad I could help!
My prof never went into this either, glad the video helped
I never comment on public place but you so much worth a complement. You are an amazing teacher.
Thank you!
Thank you!
Compliment*
@@Leah4sciMCAT and you're sexy. Just saying. 👍
Oi
I like the detail you went into they usually don’t teach this into depth, they just scratch the surface and move on. Thanks for the vid it helps even 5 years later 👍
Glad it was helpful!
6:32-7:43
Literally, in less than a minute and a half you taught me such an easy way to tell apart an L and D Fischer projection. I thought it would take forever for me to learn how to tell them apart! Thanks again!
Oh wow! So glad I helped you understand it so quickly!
I love you so much. You are so unbelievably helpful. Not only do you explain things super well, and make everything really clear, but you also maintain an animated voice which makes all the difference. The inflections and "enthusiasm" in your speech (whether feigned or genuine) really helps to pay attention and pick up the information. You are a godsend.
Thank you very much! My goal is to always make it as fun as possible :)
good luck to all of those studying for the MCAT
Yes!
💯
Of all science teaching videos I've ever seen, you make the best. Infinite thanks to you!
You're very welcome!
This is perfect, thank you! This is the only resource I've found that explains how to determine L and D
Happy to help!
This woman got me a 95% in organic chemistry 2 , and now she's carrying me in biochemistry
Awesome! Glad to help!
Thank you so much! I was panicking over having to learn this, but you made it really easy :)
You're welcome, hopefully no more panicking!
Cannot even describe in words how amazing this video is. Thank you!
You're very welcome!
This was AMAZING!!! I finally get it! Thank you!!! I always thought this was difficult to understand but you made it SUPER simple! Thank you!
I'm so glad to hear that you understand!
I am from india and i really loved and actually just grasped everything u said in minutes rather then spending hours with books and then the books made sense thanks ma'am it was really very helpful
Glad to help! :)
This is the greatest trick in organic chemistry THANK YOU
You're welcome! :)
This was so good! It really helped explaining what my professors or book could't, right before my exam. I quite enjoy the amount of examples.
I'm glad that it helped!
Leah I love you 💕 got an A on my orgo 1 and B on orgo 2 with the help of your videos and advice. You should be on the top recommended for the MCAT exam study sources.
oh wow! good to know that! Congratulations! Thank you for the nice feedback. Always glad to help :).
Oh wow congrats on acing orgo! Hope you aced your MCAT as well
literally never took organic chem and this video helped me understand the concept almost entirely omg i love u :((
Glad I could help!
oh wow, that's amazing! so glad to help
Just one watch and got the whole concept clear!!!!!!! Absolutely amaaaazzinngggg!😭✌️
YAY!!! So happy this helped you get a clear grasp on it!
i'm from Argentina and i couldn't find this exactly explication in spanish, i'm so glad to have found your video thank you very much
You're very welcome!
Glad you were able to understand in english
This is fantastically well shown, explained, and produced. Great job.
Thank you very much. Glad you enjoyed it
Thank you for the great video. I really appreciate it. I can't guess how much effort this video put into it.
You're very welcome and thank you for your kind words. A lot of work goes into this, but I love doing it!
Not only great but is actually the best thank you for the explanation 🙏🙏🙏
You're welcome 😊
Whenever i feel complications in understanding chemistry concept i refer MCAT.
You are great ma'am❤
I love the way you crack the hardest topics in the most simplest manner💐
Kudos to you ma'am🙏
Aww, thanks for that! I'm glad my resources help you to understand!
b go g
awesome!!!! i just lost my stress on covering this part of aminoacids. thankuuu soo muchhh!!!
Yay! I'm so happy to hear that, and you are very welcome!
so glad this video helped reduce your stress
Awesome! you've helped a brazilian medicine student! Thanks a lot. :)
like me.
Awesome, so glad my resources helped you!
So glad to help
Amazing lecture!!
Thank you!
Mam, while studying SN1 and SN2 reactions in school, everything made sense until my professor mentioned Walden inversion in SN2 reactions. He wrote, 'During inversion of configuration, the relative configuration generally changes from R to S or S to R, but not always.' This completely puzzled me. I always thought that R/S represents the absolute configuration and D/L (or d/l) represents the relative configuration. Am I misunderstanding something, or did I miss an important detail? I came to your video for clarity but still couldn’t fully figure it out. Please help me, mam!
I'm not familiar with the Walden inversion so I don't know. However in general an SN2 reaction will have an inversion due to the nature of the backside attack of the nucleophile
Awesome video, excellent and simple explanation, just what I needed. Thanks a lot. :)
You're very welcome
Thank you so much for making this video! You have helped me understand this topic more than 3 years of university could🥴
You're welcome! Glad it was helpful!:)
Its crystal clear... It helped me a lot to go further with this .... Thanq so much......
You are so welcome!
You're very welcome
Thanks a lot ,you explained it so clearly that I am finally going to complete my assignment , otherwise I was so worried.
Glad I could help!
Thank you! Great explanation and in a simply way. I understood in minutes. Thank you for your work 😊
You are very welcome; so glad it was helpful!
I love whoever made this channel
Thank you for your kind words!
this video has made my day
thanks to the uloader
You're very welcome!
I wish i found u years ago , thank you for this video, it really clarifies this confusing concept
So glad it helps!
Better late than never? glad it helped
Your video is amazing! Thanks for everything😊
You are so welcome!
THANK YOU SO MUCH!!! I was so lost looking absolutely everywhere and you had the easiest to understand lesson about this. I'm still a little confused but better than I was, initially when my teacher was showing it, it all flew over my head and I was so overwhelmed. Thank you!! I sent your link to my classmates as well!!
You are very much welcome! Glad to have helped and thanks for sharing the video :)
You're very welcome! Hope watching it a second time helped it all click
Thank you SO much!
You're very welcome!
@@Leah4sciMCAT ❤️❤️❤️ This has been the most helpful I can't even stress that enough 😭
You're very welcome
wow the configuration part! thanks for sharing! i totally get it now!
You're welcome! So glad this helped you clear things up!
Simply incredible, thank you
You're very welcome
Miss, I just love ❤️ to watch ur videos and do study from your lessons thanks for being my fav educator 😊
Thank you! 😃
awww thanks
Good video for quick exam prep but not very much for learning chemistry tbh. The principle and logic behind this chemistry are not explained (which is not required if you just want to pass exams especially for premeds). But I appreciated the effort. Thanks.
You're welcome
really, excellent teacher, and u r using very good methods of explanation, thanks... 🌹
you're very welcome!
thank you
you are such a genius
Thanks!
Amazing video, it helped me greatly.
Glad you enjoyed it!
Very well explained! Save me so much time!
Glad it helped!
You're very welcome!
Thank you! Finally it sunk in!
Glad it finally clicked :)
This video is really amazing! And excellent explanation..
Glad you liked it!
Thank you
This was so helpful, thank you so much for sharing your knowledge.
You are so welcome!
you're an amazing teacher!!!
Thank you! 😃
Thank you
W content. Saved me from so much grief where had you been all my life
Always glad to help!
Well done explanation!
Glad it was helpful!
thank you
Thank you so much! you explain sooo well
You're welcome! Glad I could help
You're very welcome and thank you :)
It was too easy to understand. Amazing, thank you.
You're welcome! Glad you got it :)
Too easy is hopefully a good thing
Great explanation, great explainer.
Thank you :)
THANK YOU! YOU JUST SAVED ME!
Oh wow, glad to help!
You're very welcome
Very clear explanation..thank you!
You are welcome!
You're very welcome
Thank you so much this was great Leah
yvw
You're very welcome
saved my life. not to be dramatic but would die for u omg
Wow, thanks for the offer, but I'd rather you not have to do that :)
Thank you so much for helping me with this video, ❤ 💕 💗
You are so welcome!
You're very welcome
Love this video! I love how you encourage understanding, not just memorization. I struggled with this topic a lot but you made it understandable! Thank you!
You're so welcome! I'm glad it was helpful!
Thank you! and absolutely YES to understanding before you attempt to memorize. If you memorize you forget, and then what?
If you understand you can reason your way through it because of what you truly GET
for the S-Aspartate question at 21:15 if we used the guess and check method, even if we place the +NH3 on the right side for D configuration it would still be an S, so no matter the L or D conformation it will both be S, so how can you use this method if you're trying to compare the S configuration of the line and wedge to both the L and D conformation if both are S?
I'm confused by your question. If we swapped the NH3 group with the Hydrogen and had the NH3 on the right side of the Fischer, we would have an R configuration to the stereocenter. Making a swap like this changes the configuration of the chiral center.
For help with questions like this and more, I recommend joining the MCAT Study Hall. For more details, visit join.mcatstudyhall.com/ or contact me through my website leah4sci.com/contact/
Thank you so so much for your insight! So helpful
You're very welcome!
you are very talented! great job. subscribed.
Thank you
Thank you for the kind words and for subscribing
Ur videos are amazing.. really its helps me a lott👍
Thank you for your kind words!
You're very welcome
thank you very much, you are really talented
Aww thanks so much!
Thank you :)
This was an amazing video! Thank you so much!!
You're so welcome!
You're very welcome
Thank you for your videos. You are a best teacher ever.I have followed your materials to improve my knowledge.
I have a question in Stereochemistry. There are some few videos in youtube that they mention III ry amine have a chiral center. There is a problem because when I do the ACS questions they not accepting it as a correct answer. please give me an answer If you have a free time to attend with this matter. Thank you
I'm sorry, but I don't offer tutoring through RUclips comments. For help with questions like this and more, I recommend joining the MCAT Study Hall. For more details visit join.mcatstudyhall.com/ or contact me through my website leah4sci.com/contact/
This is a tricky question, Tertiary amines are technically chiral due to their shape, but the 4th group is a lone pair which is constantly inverting and so you won't ever have just R or S. Quaternary amines on the other hand can have 4 unique subs for a proper chiral center
this is so helpful, wish I had found the video earlier
Glad it was helpful!
You're very welcome! Better late than never, right?
Why is the carbonyl and H in the wrong place at 10:30? Why are you swapping?
Correct form for a Fischer projection is to have the main carbon chain along the vertical backbone of the structure. That means, by convention, the carbonyl carbon should be on the vertical line and not the horizontal. One swap of the carbonyl and hydrogen changes this to follow convention, but then we have to do the second swap to maintain the R configuration of alanine.
@@Leah4sciMCAT thank you for taking the time to explain :)
Late reply on my part, but you are very welcome!
A very useful video!
Glad you liked it!
i like it .....easy tricks to solve problems
I'm glad you like it!
You are the best ... thank you so much
You're very welcome!
You're very welcome
but we should draw perfect fischer by placing most oxidised carbon on top and maximum carbon vertically right ?
Yes, correct. For more detailed help on drawing Fischer projections, make sure to see my series at Leah4sci.com/Fischer
best voice EVER
Thank you for your kind words!
you saved me...... thank you.......♥♥♥♥♥
You're welcome!!
Can you explain how you know the H is coming "forward" even though it just appears to be in the plane?
If speaking about the Fischer projection (looks like a flat cross molecule), then groups on the horizontal line are coming out of the page, by convention. While groups on the vertical line are going into the page, by convention. For more about Fischer projections, what they mean and how to draw them, make sure to see my free tutorial series at Leah4sci.com/Fischer
I think you meant to say that oxygen has more priority than nitrogen since we are going by atomic numbers not the other way around. So the carboxylic group will be number 1 and the ammonia will be number 2.
The carboxylic group has a carbon (not an oxygen) directly attached to the chiral center, and carbon is lower in priority than the nitrogen in the amine.
Oxygen outranks Nitrogen, however in a carbonyl group it's the Carbon we're comparing to Nitrogen and so the amine outranks the carboxylic acid
Why did you skip the two hydrogens of the CH2-SH group? Wouldn't the Carboxyl group have higher priority due to the amount of bonds to oxygen?
Both Sulfur and Oxygen are directly bonded to a carbon on the chiral center. If Sulfur outranks Oxygen with its atomic number, the number of bonds to Oxygen are irrelevant. Sulfur is the winner and would be higher priority.
Thanks ma'am
You're welcome! :)
you're welcome
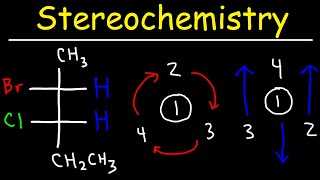
Thanks for the video, I just don't get when R and S is reversed, like in 12:00. If the lowest ranking group (normally hydrogen) is to the right of the Fischer projection you reverse it, but if it's at the top (like at 9:50) or bottom then we don't reverse it?
Yes that’s right because the H is already in the back in contrary to when it is on the side and facing to the front. Hopefully that was helpful :)
Since we want the lowest ranking group to be at the back of the molecule when assigning configuration, we must reverse the designation if that hydrogen falls on the left or right of a Fischer projection. Because, by convention, the left and right groups are moving up and out of the page. If the hydrogen falls along the vertical spine of the Fischer projection, then it is already pointing behind the page, or to the back of the molecule. Therefore, no reversal of the R/S configuration must be made.
To learn more, watch my Chirality and Stereochemistry series (especially the Swap method video) at Leah4sci.com/chirality
@@Leah4sciMCAT @Milo K thank you both !!
I cover stereo of Fischer extensively here: leah4sci.com/fischer
Splendid video!
Glad you enjoyed it!
You go girl, this is amazing! Thank you!
Glad to help! You're welcome :)
You're very welcome
Can you reiterate how exactly you know when to change the direction of our loop when connecting 1,2&3? Is It only when the hydrogen is coming out of the paper?
Go back to this series: leah4sci.com/chirality/
I cover this extensively here: leah4sci.com/chirality
I am stunned
Thanks!
you are great..thanks
Thanks, and you're welcome!
Here I am studying for my 2nd biochem test and this is the first time I'm learning R & S aren't the same as D & L? my professor literally told us that they were - that L was R and D was S. Gotta love paying thousands of dollars to be given wrong infomation D:
Ouch! Sorry to hear that!
Oh wow, yeah my biochem professor also tripped up on R/S basics, we had to correct him
That was just WOW
Thanks!
Thank you :)
Thanks
You're welcome!
Thanks, you are a life saver!
Now I have to move on and deal with E/Z, though. -.-
Glad I could help!
Is this what you are referring to?
leah4sci.com/cis-trans-and-e-z-geometric-isomers/
Ah, yeah, exactly! Thank you :D
You're very welcome! I cover that in detail as well
Thank you so much
You're welcome!
you're very welcome
Very very helpful!
thank you!
Thank you
awesome video, thank you so much
You're very welcome!
You're very welcome
Can somebody explain why she does the second swap (during 10:32) ? I know she said to "maintain the chirality," but can somebody explain that please! Thank you:)
At this point in the video, we are trying to transform the Fischer projection of R-alanine into proper form where all carbons would be along the vertical backbone of the molecule. That means we need to swap our carboxyl group to the top-most position, so that it falls along the vertical.
When we make that swap, we also need to swap two other groups in order to maintain an R configuration at the chiral center. If we didn't make a second swap, we would then have the S configuration of alanine. Which is a different stereoisomer of our original molecule.
Thanks ❤
you're welcome
Leah4sci is bae af
Thanks! I'll take the compliment. :)
Thank you soo much
You're welcome!
YOU ARE AMAZING THANK YOU❤❤❤❤
You're very welcome!
You're very welcome ♥️♥️