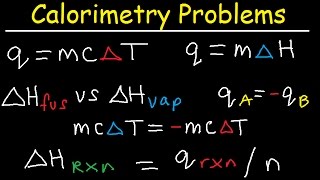
Find the Heat of Dissolving (Delta H, Dissolution)
HTML-код
- Опубликовано: 5 фев 2025
- Put a solid into water ... temperature changes...what's the heat of dissolving?
Find q with mΔTc, and divide it by the number of moles of solid you put in.
Make sure your SIGN is right. Negative ΔH = Exothermic = Temp went UP.
Positive ΔH = Endothermic = Temp went DOWN.








you taught me more in 5 minutes than my chemistry teachers ever have. God bless you.
literally bless your life you made my inquiry lab make sense i am going to cry
Jesus Christ! You, sir, are a life saver/genius
May I ask a question? In computing for heat of solution, should we add the mass of solute and the mass of solvent together to have a mass of solution? Or only the mass of solvent (water)? Other sources say that both masses of solute and solvent should be added together. Thank you
Can someone smart please answer this. I'm begging, for a friend of course
I am in this dilemma rn
Waw the entire 5min26 are important in this video, even the last second since I got the endo/exo thermic explanation exactly at the end. Great video that explains it all. Got 100% in my lab Thanks
What a GUY. u deserve an applaud 👏👏👏👏
Thanks dog, 10 years later and this video's still saving my ass. Means its a good video I suppose.
Oh sweet jesus, thank you for uploading this. I am saved from failure on my test tomorrow :D
7 years later, curious if you passed the test?
@@SurgicalSituation still waiting 10 years later
still waiting 10 years and 12 days later
BOOM indeed. Thank you, good sir, for snatching my grades from the jaws of oblivion
God bless you.
i love you man im literally crying rn
BOOM THANK YOU SO MUCH. SO INFORMATIVE AND CONCISE.
You just saved my life thank you!!
Thanx dude 🙌🏼🩵
Why don't you use 55g as the mass since it has dissolved into the water?
Because 5g of salt is too small to cause a change in the volume of the water. If you had 50ml of NaOH and 50ml of HCl then that would cause a change in mass. The total mass would be 100ml or 100g
take the derivatives of C, then measure C and make a time graph in which you take za Integrals, if za values are lower zhen 0, means you don't use 55 g as the mass!!! if greater then you do use the mass, this this case it was lower then 0.......its not as complicated as it seems, douches from China
@@talhajat3301 Douches is a term that means "penis" so...
@@jqyhlmnp not true it's something else might wanna look it up
@@courtneybrowne4773 Your second sentence is describing mixing two SOLUTONS together not adding a salt to water. There is a difference. Also, my Chemistry teacher ,who got her PhD from Georgia Tech in Biochemistry, made all of us students feel stupid for not calculating the mass of the water and the mass of the salt we used (which was 5.00g for both trials) when calculating the heat for the reaction.
Thank you! This was very helpful!
My hero!
thank you so much I've been struggling in AP chem
Thanks so much for this video. Made a lot more sense.
hey thank you so much I wish I found this more earlier!
Thank you so much!! Saved me!!
love you!!! you are the best!!
Omg thank you very much!
Top notch explaining right there
THANK YOU SO MUCH!!!!!!!!!
you are a god
How do we dow this for per gram?
where do u get the 4.184 j/gC from? please help!
I thought that to do this calculation, all masses must be converted to kg? Is this right?
and the specific heat capacity of water is more
well never the less this video helped heaps, cheers man
Why would you convert mass to kg? And no, the SHC of water is in fact 4.18.
helpul thankyou!!!!
wouldnt you convert the temperture from c to kelvin?
this is a good question since specific heat is J/g*K
@@alvincomputer if you look up "what is the specific heat of water" it is 4.184J/g* degrees Celcius. However, you could look up the specific heat of water in Kelvins but, for this example, the temperature change was measured in celcius therefor you would want the specific heat in celcius so Celcius canceled out.
Why is 50mL of water 50g of water???
+Jalen Stricklen water is 1 g per ml. that is how the metric system is built
g = mL for water
what mole are you using?
very helpful
so helpful
Very good video, I may be wrong but I think this reaction wasn't in fact exothermic, but rather endothermic. If the heat went UP, then energy was being absorbed and therefore the deltaH would in fact be positive.
Thank you i was confused on that as well
I believe he was talking about the dissolution of the solid, the solid releases heat, that's why the temperature of the solution goes up.
you mean 50 mL of the water?
1mL= 1g
god thank you
This is incorrect the mass of solvent must be added to the mass of solute used. This is misleading kmt
2:57