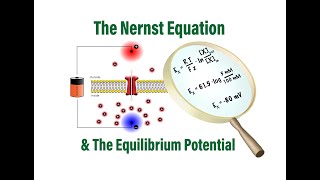
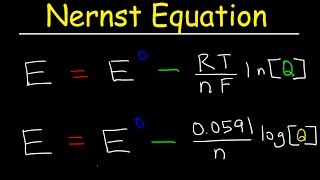
The Nernst Equation
HTML-код
- Опубликовано: 15 апр 2012
- For students of analytical chemistry (quantitative analysis). This video works through a sample calcuation that uses the Nernst equation. The video also shows where the equation comes from, in terms of basic chemistry/electrochemistry theory.







Me and my kitten enjoyed watching this video, simplified! Thnx:)
Best explanation i have ever seen. Its the first time i understand it.Please make more chemical videos. Thx from Germany
An amazingly put out video. Straight to the point and well set out, no confusion. Thanks you!
Are you high😮😮😮😮
great brain power. Thanks so much. Best presentation ever of this equation.
i want a teacher like u with more understanding ;-)
This was very helpful, Thank you!
amazing video.....simple and clear
amazing!
It's a lot of information. Would have been great to see practice problems and have this video broke up a bit into different segments.
Thank you for what you did do though!
Thank u very much
Good video!
Thank you so much for this video. So well explained!
those...GASES!
At 12:28 in the vid; Q = [(C)(D)/(A)(B)], why does "d" go in the denominator?
Shouldn't the anode half-reaction have a negative standard reduction potential? (-0.535 V) since it is oxidizing and the equation should be flipped.
great
Thanks mate, you're a lad
Best than lectures
Can anyone explain why do we use the gas constant in the formula when we don't have to deal with any gas?
+Enida Nushi Hi it refers to the basic equation of chemical potential in thermodynamic i guess
Just a little doubt.In the end, at the nernst eq., why did the "n" had to be 6 (from the I), and not 14 (from the H+)??
Migas Almeida The n is the number of moles of electrons transfered.
Infinite likes from me....