How to Draw the Lewis Structure for the Sulfate Ion
HTML-код
- Опубликовано: 22 янв 2025
- A step-by-step explanation of how to draw the SO4 2- Lewis Dot Structure (Sulfate ion).
For the SO4 2- structure use the periodic table to find the total number of valence electrons for the SO4 2- molecule. Once we know how many valence electrons there are in SO4 2- we can distribute them around the central atom with the goal of filling the outer shells of each atom.
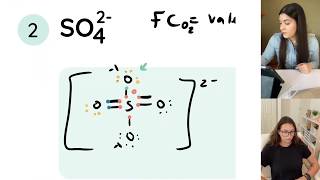
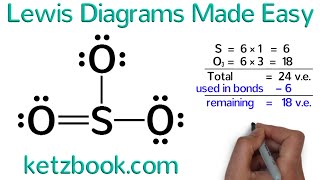
In the Lewis structure of SO4 2- structure there are a total of 32 valence electrons. SO4 2- is also called Sulfate ion.
Note that SO4 2- can have an Expanded Octet and have more than eight valence electrons. Because of this there may be several possible Lewis Structures. To arrive at the most favorable Lewis Structure we need to consider formal charges. See how to calculate formal charges: • Formal Charges: Calcul...
Also note that you should put the SO4 2- Lewis structure in brackets with as 2- on the outside to show that it is an ion with a negative two charge.
---- Steps to Write Lewis Structure for compounds like SO4 2 ------
1. Find the total valence electrons for the SO4 2- molecule.
2. Put the least electronegative atom in the center. Note: Hydrogen (H) always goes outside.
3. Put two electrons between atoms to form a chemical bond.
4. Complete octets on outside atoms.
5. If central atom does not have an octet, move electrons from outer atoms to form double or triple bonds.
---- Lewis Resources ----
• Lewis Structures Made Simple: • How to Draw Lewis Stru...
• More practice: • Lewis Dot Structure Pr...
• Counting Valence Electrons: • Finding the Number of ...
• Calculating Formal Charge: • Formal Charges: Calcul...
• Exceptions to the Octet Rule: • Exceptions to the Octe...
Lewis Structures are important to learn because they help us understand how atoms and electrons are arranged in a molecule, such as Sulfate ion. This can help us determine the molecular geometry, how the molecule might react with other molecules, and some of the physical properties of the molecule (like boiling point and surface tension).
Chemistry help at www.Breslyn.org







I FINALLLLLY UNDERSTOOD LEWIS STRUCTURES!!!!!!!!!!!!!! THANNKS SOOOOOOOOOOOO MUCH!!!!!!!!
I can understand your excitement
@@laxmankoirala405 😂
😂
Same😅✋
All teachers should start explaining like you. What an amazing explanation
Maybe we should just watch him 😅
I slept through two 1hr 50 min lectures and it took you all of 4min and 18 seconds to bring me to understanding. If all classes were like this, I could obtain my bachelor's in a year's time.
Glad I could help with SO42- and all the best in your studies. --- Dr. B
In response the question by Minh ...
Sulfur is in Period 16 (also called 6A). Therefore it has six valence electrons, not four. I think that should solve your problem.
Note also that Sulfur is below Period Two on the Periodic Table so it can have an expanded octet (more than 8 valance electrons).
--- Dr. B
Wayne Breslyn Thanks for the helpful videos! Just to make sure, in your comment, you said Sulfur is in Period 16. You meant group 16, right?
Sir, what other elements than those under the period of 2, could hold more than 8 electrons? Can all elements under the period of 2 extend their octet? (°-°) And why can they extend more than 8? Sorry if I'm asking a lot, this wasn't discussed in our class.
Thanks, Dr.B, This is a very good presentation. However, I want to add the following for better understanding of the problem.
"Elements which have vacant d-orbital can expand their octet by transferring electrons, which arise after unpairing, to these vacant d-orbital e.g. in sulphur. In the excited state, the sulphur has six unpaired electrons and shows a valency of six e.g. in SF6. Thus an element can show a maximum covalency equal to its group number, e.g. the sulphur shows maximum covalency of six."
I hope I am correct. Thanks once again.
Yeah ok
Because d orbitals are involved period 3 and below. Also, yes.. all elements period 3 and below can have an expanded octet.. not just sulfur. Cheers
I'm from Germany and our teachers explained the lewis structure completely different than you did but your way is much more simple, thanks it kinda helped
I seriously love watching your video! They help me very much when I'm Struggling in class. Thank you for making these amazing videos !
Those dots are refreshing.
These are so helpful! Never stop teaching! I watch these after my chem class and they're so helpful every time. youre a life saver!
That's a great learning strategy to watch them after class! --- Dr.B
You are the best teacher ever!!! Never have I seen a teacher this good
Thank you! Most appreciated. --- Dr. B
THANK YOU SO MUCH I WAS SO CONFUSED BEFORE THIS VIDEO BUT YOU BROKE DOWN LITERALLY EVERYTHING THANK YOU SO MUCH
Online chem is no joke. I'm so glad i have you Dr B or i would be a lost soul.
Me who's watching this video after 10 yrs😅
11 years 😂
After 11 years ha ha😂
11 yrs bro
Bro!...thnks alot...you did what our professor was not able to do for hours:-)
Happy to help!
Wow these are advanced Lewis structure examples! Thank you for sharing!
You are right, SO42- is a bit of work with the formal charges! --- Dr. B
Thee Best video ever,best explanation ever 👌 pls don't stop making videos it helps 😉🤗
Thanks for kind words and encouragement! --- Dr. B
If my teacher taught legibly and lightly like you, my Chemistry result would be better than now. '''
Any way, thank you, Dr.B . You get my inspiration up :)
Thanks for the kind words and encouragement. Glad I could help! --- Dr. B
Could you accept my friend request on Facebook? Thank you!
+Nguyễn Minh Vũ lol =D
I love the way this man teaches , i understand it know
Excellent!
Really you are aswsome
This is the best explanation I have ever seen
KING COBRA Thanks, King Cobra. Glad I could help! --- Dr. B
You have explained in a good way👍
Thanks a lot 😊
excellent job Dr. B, i would otherwise be unable to understand why some other lewis structures are out of my expectations
That was great. I saw that sulphur and oxygen are on group 6a so i couldn't comprehend where the 2 extra electrons came from. Now i realize you have to consider valence electron capacity. its a new can of worms to consider, but you set me on the right path for comprehension; thanks!
just rewatched your video. I think its a matter of understanding the rules for bonding and non-binding to get your ionic charges.
Yep, you got it!
Trick :
Oxygen with a single bond has always -1 formal charge
Oxygen with double bond has 0 formal charge
Oxygen with triple bond has +1 formal charge
Pls tell me there's no exception and this works for all
Your videos are so straightforward and helpful. My new favorite Chem study aid! Thank you!
Happy to hear that!
Thankyouuuuuuuu....sooooo. muchhhhhh...... finally I know what is lewic structure....thankuuuu
Finally I found the vedio which l look for it😍😍😍😌😌😌
Excellent! --- Dr. B
Thank you Dr B! Really good and clear explanation there :D
Thanks Dr.b.I was actually misunderstood.Now I'm all cleared.
Excellent, glad I could help! --- Dr. B
I didn't think chemistry could be so sweet😍
i found this educating in a more simpler way. thanks
Thank you very much! Greetings from Brazil!
No problem! Glad I could help with the sulfate ion! --- Dr. B
Can't get it ...
You provide a great resource with these videos! Thank you for posting :)
Thank u so much sir
This was a very good explanation
You are most welcome! --- Dr. B
Thank you. You made it easier for me to understand.
Glad I could help with SO4 2- , it can be a challenging Lewis Structure. --- Dr. B
For the SO4 molecule with all single bonds, only 4 of the valence electrons of sulphur are participating in bonding right? One for each oxygen atom. So won't the number of non-bonding valence electrons for sulphur be 2?
Also, in the sulphur oxygen double bond, three of the shared electrons are from oxygen? Only one is from sulphur?
Rosh Varghese Another question - if none of the valence electrons of oxygen participate in bonding, where does the other electron in the covalent single bond between oxygen and sulphur come from?
Thank you! I really apreciated your way to teach! It's amazing!
You're very welcome!
Would this not also require several resonance structures to show that the double bonds could be either on the side oxygens or the top and bottom oxygens or a top and side oxygen etc?
Yep, that would be the case here. Also note that drawing the structure with only single bonds also is a viable resonance structure as well - however it's not as important as the others you mentioned since it's formal charges are higher. - Dr. B
I really need help,
In the first structure where all are single bonds,
Is that a valid Lewis dot structure,
And if it is a valid Lewis dot structure, then will the other structure be a resonating structure of it?
1:25 sorry i don't understand, Isn't there are two electrons not involved in bonds?
S have 6 VE and it only form 4 bonds with O
Here there are two double bonds and two single bonds on the S. So it has an expanded octet with 12 total electrons.
Thxx you I understand now
What is it’s Electron Group Geometry? Shape? And is it polar or non-polar?
Here's a start:
ruclips.net/video/f32ra5yjG0s/видео.html (shape)
ruclips.net/video/DC2O3-sA68A/видео.html (polarity)
Thank you very much for explaining some complicated topics
No problem, SO42- is a bit involved to write the Lewis Structure . --- Dr. B
thank you sir ,
you explain so good
Your video always helped me!💕
Thank you so much .i had problem at this subject but now completely has solved
I got the same structure but what's the bond order? I got 2 but another site says 1.5 and i want to know why that is
If you use the Lewis structure for SO4 2- with two double bonds and two single bonds the bond order will be 1.5 (6 pairs of bonding electrons divided by 4).
The folks at UCDavis have a decent page explaining how to determine bond angle (chemwiki.ucdavis.edu/Theoretical_Chemistry/Chemical_Bonding/General_Principles/Bond_Order_and_Lengths).
All the best,
Dr. B
Thank you so much!
Amazing explanation, thnku soo much
Thank you!
can someone explain me if the blue oxygen is sharing 1 electon then it’s supposed to be 5 non bonding val electrons, why it’s 6?
Do you know what the lewis structure of potassium aluminum sulfate (KAl(SO₄)₂) better know as potassium alum is like?
do you know What is the formal charge of the central atom in the Lewis structure of SO42- that obeys the octet rule?
S can have expanded octets so it doesn't have to obey the octet rule.
Exceptions to the Octet Rule: ruclips.net/video/Dkj-SMBLQzM/видео.html
Hi Rayma. Any element in Period Three (row three) on the Periodic Table can have an expanded octet. Sulfur is in Period Three so it can have more than eight valence electrons.
In the SO4 2- Lewis structure Sulfur has an expanded octet in order for the formal charges to work out.
- Dr. B
your a great teacher
Thanks! --- Dr. B
Why you wrote that the number of nonbonding val electrons of sulfur is 0? If there are 6 valence electrons and 4 of them involved in bonding, then 2 electrons are in a lone pare, although it is not indicated in your picture. I do not get it
Bruh my teacher didn’t teach formal charges but expects us to know how to draw Lewis structures properly. Anyway tysm!!!
sir i dont understand oxygen valency is 6 after bonding it must get 5 around it why u kept 6 dots
It needs 2 to complete it's octet
isn't S in the second period of the table?
S(16)= 1s2 2s2 2p6 3s2 3px2 3py1 3pz1
We can see that S has two lone pair electrons. Why we didn't count those?
what is this application? does anyone know?
Is that coordination bond exist
+Wayne Breslyn, what is the difference between formal charge and net charge. For instance when we talk about Oxygen being 2- what is that defined as formal net or is that an ion?
sir , may i know that how we will gt to know that how many double or single bond will be present in any molecule like your H2SO4 or PO4^2-?is guessing the only way?
How can we add in the charge at the begininng if we don't even know it
There are two ways I can think of. First, if you're told you have the sulfate ion that is always going to be SO4 2- . So if you're asked to draw the Lewis structure for sulfate it will have a minus two charge. This one is worth memorizing since it comes up often.
If you see something with an ending of -ate you'll want to look it up in an ion table.
The other way you would know would be when it's actually stated in the problem. I ask my students to draw the Lewis structure for SO4 2- not just SO4.
---Dr. B
thanks very much sir, however my real question is very simple. how do you get the charge if it's a covalent bond and you only know about the SO4. if you may can you please make a video about it.
I dont get where the two additional electrons come from
You can sort of think of it this way. They would come from whatever the sulfate ion was bonded to before it became and ion. For example when Na2SO4 dissolves in water there are two Na+ ions and one SO4 2- ion. The the 2- on the sulfate came from each Na atom losing an electron. Does that help? --- Dr.B
@@wbreslyn Yes it did help, thank you! I have another question though: How can you tell when a free pair of electrons is going to turn and function as bonding electron pair ? Does this question make sense ? Like for example in H2SO4. I could've drawn the Lewis structure with every atom making a single bond (in this case the 2 free electron pairs of sulfur already turned into a bonding electron pair between the oxygens) and then just write down the formal charges. But the structure has two double bonds between the S and the O, meaning that one free electron pair of the oxygen also turned into a bonding electron pair, what makes the double bond. In this case there are no formal charges which is somehow that what we want, but in the end I never know when i can apply something like this.
I hope you can understand my question, I tried my best formulating it since English isn't my main language.
Excellent video, I understand 😅
Nice! --- Dr. B
According to the new structure u drew, Sulphur has 12 valence electrons instead of 8. Is each element not meant to have 8v.e so as to complete the octet structure?
The Octet Rule is a very general rule. Many elements can have expanded octets (non-metals in Period Three and below on the Periodic Table). Take a look at these videos:
• Exceptions to the Octet Rule: ruclips.net/video/Dkj-SMBLQzM/видео.html
• The Octet Rule: ruclips.net/video/6Ecr7m-0E0E/видео.html
---- Dr. B
Such an amazing helpful video
So we can have two structures of Sulfate that make it anionic (-2) right ?
If you mean resonance structures, there are several possibilities:
en.wikipedia.org/wiki/Sulfate#/media/File:Sulfate-resonance-2D.png
All are -2.
--- Dr. B
LOL, I didn't' know it was that way, in high school they never show me that, hmm... Some times I think the educational system underestimate the teens intelligence. Thinking on those resonance structures is more easy.
I agree that high school often underestimates teens intelligence! --- Dr. B
would the first way be considered correct?
It's correct in the sense that we've used all the valence electrons for the SO4 2- Lewis structure and that all the atoms have octets. So you could consider this a resonance structure but not the primary one. The second one is the more likely Lewis structure because it has formal charges closer to zero.
However, it sort of depends on you teacher. Many high school teachers will accept the first Lewis structure (but maybe not high school AP teachers).
- Dr. B
What would be the molecular geometry of the oxygen molecules?
which elements usually have extended orbits i mean we can add more than 8 electrons
Elements in Period (row) Three or below can have extended octets. They don't always, but can. But you'll want to check the formal charges to be sure. ---Dr. B
Which elements will have expanded octet ???
Here you go!
The Octet Rule: ruclips.net/video/6Ecr7m-0E0E/видео.html
Exceptions to the Octet Rule: ruclips.net/video/Dkj-SMBLQzM/видео.html
---Dr. B
Amazing explaination! thanks man!
If the Sulphur is sharing two single bonds, a dative and double bonds with oxygen atoms. Would tht b considered correct?
Will the teacher approve the first method? I think it will be too complicated for my son
How did you know the shape to follow? Can it be a tetrahedral?
Take a look at ruclips.net/video/f32ra5yjG0s/видео.html for a video I did on the shape of SO42- (and your're right - it is tetrahedral). --- Dr. B
Excellent explanation sir thanks a lot
You are most welcome! --- Dr. B
Thank you again professor!!
No problem, glad I could help! --- Dr. B
In a single bond do only sulphur shares ? Oxygen electrons don't share in VB a single bond here ? How many electrons sulphur share in a single bond ? Valence electrons of sulphur of has 6 then how it can share 8 electrons with oxygen
Hi , I have question. How is the formal charge determine the drawing of the structure? If it's -1 charge then so how does it affect the structure ? And same goes to 0. Please help thank you ❤️
Good questions. If there is a -1 charge on an ion that would mean that the total formal charge for the atoms in the ion would equal -1. If it's zero then they all need to add up to be zero.
Take a look at these videos for how to do formal charges:
• Determining Formal Charge: ruclips.net/video/vOFAPlq4y_k/видео.html
• Formal Charge Practice Video: ruclips.net/video/-9f4H0puVzc/видео.html
--- Dr. B
According to the structure blue Oxygens have 5 non binding electrons, it means their charge is 0. Please explain.
sir if you are online please help me how to make resoning structures
Thank you sir it helps me too much please make videos on knowing stablity
This video was extremely helpful, thank you!
Thanks, glad I could help with SO4 2- ! --- Dr. B
Your a lifesaver :)
Excellent sir superb super 😈
Hello,
Why is it necessary for the formal charges to be as close to 0 as possible?
actually it's a rule they made to find the Best and more stable Lewis structure
does it follow octet rule?
Thanks! You explained it very well!
I’m Korean student! It’s the best..
Great video but I'd like to suggest that more contrasting colours be used other than dark green and blue hard to tell the difference, although it is easier in full screen mode!
what would be the overall charge for this? because it would be "2-" but I don't know how
Yep, the sulfate ion (SO4 2-) has an overall charge of -2. --- Dr. B
thanks, but how do you determine that from the formal charge, because a question required the calculation of the overall charge after the summing of the formal charge? so I summed the formal charges and "i got 0+0+-1=-1" as the overall charge.
You are very good Sir!!!. Thanks.
super explanations..amazing..
Thank you so much 🙂
And why are there 12 electrons for sulphur??
Hey , oxygen has 6 valence electrons, so can the blue oxygen in your diagram have 3 lone pairs , it's like oxygen had 7 valence electrons out of which 6 remained with it as lone pairs and one was used in bond formation with sulphur ? Please help me .
Oxygen alone will have 6 valence electrons. So it will share with another atom to have eight. In the SO4 2- lewis structure is shares differently (single or double bonds) so that every atom can have an octet while using only the valance electrons available for the molecule. Here's more than you ever wanted to know about the Sulfate ion ...
en.wikipedia.org/wiki/Sulfate
--- Dr. B
I had a chemistry test recently where I had to do the Lewis Structure for Sulfate. I thought that was a really easy question as I had watched this video the day before, so I drew the sulfate as it was in this video and my teacher marked it incorrect. Is my teacher correct or mislead?
It's likely they didn't take into account formal charge. Your best defense is to show them the structure with the two double bonds from a respected source (en.wikipedia.org/wiki/Sulfate works well or pubchem.ncbi.nlm.nih.gov/compound/Sulfate). --- Dr. B
Why does the sulfur go in the middle when oxygen is more electronegative? I've been taught that the most electronegative atom goes in the centre, so it would be 3 oxygens on the outside along with one sulfur, and then an oxygen in the centre, why not?
+XAnthony1592X Actually the least electronegative atom goes in the center (almost all the time). Hydrogen is an exception and always goes on the outside of lewis dot structures. --- Dr. B
Yeah, I was reading my notes wrong... Thanks heaps though :)
I still don't get why Sulfur is stable with 12 valence electrons. It doesn't make sense according to my elementary level understanding of chemistry.
Have I misunderstood that sulfur seems to have 12 valence electrons counting the shared ones? Or is my understanding of chemistry fundamentally wrong in this case?
The octet rule in chemistry is a very general rule. There are many exceptions including expanded octets. Take a look at this video on the exceptions.
Exceptions to the Octet Rule: ruclips.net/video/Dkj-SMBLQzM/видео.html
--- Dr. B
Thank you Dr. B!
Thanks it's very helpful 😊
Thanks! --- Dr. B
I understood it immediately.