ELECTROCHEMICAL CELL || HINDI EXPLANATION || ELECTROCHEMISTRY || 12TH CHEMISTRY
HTML-код
- Опубликовано: 22 янв 2025
- welcome to visual learning
ELECTROCHEMICAL CELL || HINDI EXPLANATION || ELECTROCHEMISTRY || 12TH CHEMISTRY
This channel provides educational videos for science and technology for school board education.
Animated videos for school education available in both Hindi and English.
Join our membership and access all premium videos:
Membership Features:
1.Exclusive videos only for members.
2.Animated series of all topics from 9th to 12th class
3.Videos are available in English and Hindi
4.Members chat room after membership.
5.Priority to comments for members
Membership fee: 599/-
See what you will get:
/ @visuallearning247
See Content for Members:
• Members-only videos
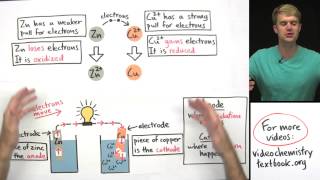








It gives better understanding than two hour of lecture . thank you so much
I don't like chemistry at all but your explanation is literally amazing.
It is my favourite subject
Oukat me rah sabaka baap he chemistry . Nikal 😡
❤❤❤❤
@@nm.gulps.true😂
@@chadslayss Both are equally good. But job wise chemistry has good scope in industries (pharma, chemical, leather, many more)and laboratories too.. u may jump for research, school/college lecturer also. Incase of physics it is restricted to research and few jobs in laboratories, schools and colleges.
So stop being a smart as$.
Bhai aap to kmal ho aacha kam kr rhe ho लगे रहो आप का चैनल jrur grow hoga or thanks lot bro bhot hi genuine explain Kiya h aap ne 👌👌👍👍👍👍👍
Best Explanation Ever
my school teacher had taught something opposite
but u have taught it right
thankyou so much sir
Amazing explaination❤❤ everything is crystal clear❤❤👍👍
Too crisp information, just to the point. ❤
I wrote each line in my noted.
Thankyou, wishing more growth❤
Bhai mai 1 mahine se smajhne ki kosis kar raha tha lekin nhi smajh aa raha h tha lekin tumne shirf 5 min me samjha diya...wow lovely animation
I saw an increment of 1k in 3 days.This is a time when students watch video because it board times.
Yes afkors
@@alibrand619bhairy haardh englis
@@pulkitgupta7164 you bhairy komedy guy
Thank you so much 🙏 for this amazing explanation ❤ye topic mujhe bahut achhe se smjh aa gyi 😊
Best video for a quick recap...love it❤ keep making such more
Wow nice explanation 👌👌👌
Very good explanation sir thank you for your help sir 🙏👍
Wonderful explanation ☺
Thankyou sir it is so helpful 🙏
I was unable to understand this in all other lecturea this video gives best understanding thankuhh
Which board
Bhot bhot aacha explain Kiya ❤❤❤❤❤❤❤🎉🎉🎉🎉🎉🎉🎉🎉🎉🎉🎉🎉 ....thank uuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuuu
Thnx sir very best Explanation 👌🏻
best explanation ❤❤
Waooooooooooo bohat achi samj aie hy
Thanks for your information ℹ️🤠
Bahut achha explain kiya hai 💕
Thank you very much sir Allah may bless you
Thank you lot❤️
Outstanding explanation 😮😮
Thanku so much for such a wonderful video
best explanation 👌👌
Kaun kaun meri tarha chemistry ke exam ke ek din pahle ye dekha raha haii attendance please😂👇👇
Mai
Me bhi😂
@@vermakushagra ☺😅
@@MohitKumar-nv2mz 😄
Present 😅
Thanks a lot. This was extremely helpful.
Thank u sir I understand each and every thing for my practical ❤❤❤
Hats off to you sir , thank you so much
*Amazing Visual Learning*
Uncle is the best ,bahut acha btaya apne uncle . Thankyou uncle😁😁😁
Uncle🙄
Please make more and more description........with many many youtubers and so on.....
thanks for your suggestion
Very impressive 👍👍
Well explain sir😊
Best explanation
Thanx a lot sir.....😊
Thank you very much ❤
Thanks a lot 👍👍❤
best explanation...
I understand sir ❤❤❤
Bhai bhot accha kaam kr rhe ho
Amazing👍😍
Amazing 👏 🙀 😯 😮 😲 👏 🙀
Thank you for your great explained
Thanks a lot😊
Zeher samjhaya bro
Amazing👍
Thank you ❤
Maja aagaya bhai 💝👍
Excellent 👍👍👍👍🙏🙏🙏🎉
Why do electrolyte ions from salt bridge react with ions in solutions of half cells even though the electrolyte is inert?
they dont react or combine. the excess of positive zinc ions on anode simply attracts the negatively charged ion(cl- if kcl or so42- if k2so4) to maintain the electrical neutrality.
@@Nitya-bw7kcsame same😂
Very nice explaination keep it up and thank u
What r u doing now??
Thanks ❤🙏
Very nice explanation sir
Then your channel will famous.....
Awesome explanation
Very Very thanks
Best teching 🎉
Nice 💯💯
Nice explanation 😎
Nice.....
Mst explanation sir😊😊
3:18 par( LOAN) ke anusar Anode pae to -ve charge hota hai n..???🥲
Bhai sab...
Maza aa gya😅
Sir nice 👌 3d video
Thanks a lot
you are gineus boss ❤❤❤❤
Ultimate video
Thanks sir
Sir lead acid k bare Mai bi bataiye plz
Amazing....👍
Thank you❤
Thanks 🙏
Very very very wonderful explanation sir but sir I having a dought can I ask
Yes?
@@visuallearning247 sir why yu had used the potassium on salt bridge from cuso4 solution beaker
Thanks🙏🙇🙏🙇 sir
Thanks but i have a question
Zinc metal ko oxidize kon kartha hai?
Salt bridge
Sir eloktrod kis dhatu ka bna hota hai
Vivek sir said too see this video
Electrical energy converted into chemical energy ❤
thanks sir
Best explanation
Thanks a lot
Sir aapne kaha ki Salt bridge reaction me bhag nhi leta but vo to last me kar raha hai please reply...
I understood धन्यवाद
thanks for your feedback
please support this channel by sharing these video to other
Thanks 🙏 sir
Wow nice explanation
Thanu sir
Thodi der mai exam hai 😌🥱
Moat ka khel
Mara Be😅
Bhai fail hua ja pass
Good🙏
That s amazing
Good 👍
Examination hall mai dikh raha hu .... 💀
Kessa huwa exam
Let’s have boxing match between Paresh sir and Vivek sir
thanks Sir
Ek time pe zn electrode complete end hojata hai???
Ha
Haa ..or issiliyye ye cell exist nhi kregi
Ha ..
Isliye hame cell change karna padta hai
Electric energy jab tak pass karayenge jab tak
Next beaker mae cu2+ kaise aye the
Super ❤️❤️
thanks a lot
sir Isse chapter aur bhi videos banyie na please please please 😢
Wonderful ❤
I love animated study
❤ good