Draw the Orbital Overlap Diagram of O2 (Oxygen gas)
HTML-код
- Опубликовано: 16 окт 2024
- O2 is usually considered to be DOUBLE bonded.
This means each oxygen atom is sp2 hybridized; I draw the electron configuration diagram in this video.
Then, I show you how to draw the sp2 hybrid orbitals (in a trigonal planar arrangement) and the unhybridized, leftover, 2p orbital which is above and below the bond axis. These unhybridized orbitals overlap side-to-side to form the pi bond.
Check me out: www.chemistnate...


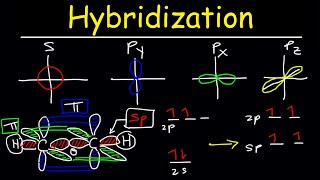






I find it quite surprising that it is often the unpopular videos that clarify all the doubts, I surfed the web and couldn't find the orbital diagram of O2 molecule.
Thank you.
:)
He drew diagram for singlet O2, an excited state. Ground state O2 is triplet, aka a diradical.
This was exactly what I was looking for. I couldn't figure out how the orbital diagram would be visualized onto an overlap diagram and this was amazing. Thanks!
Very clear and thorough explanation! Thank you very much, quite appreciated. Not to mention it's the hand acting for me.
you made something so complicated literally so simple. thank you so much. may God bless you.
Thank you!!!! This was such a good, clear, representation!!!
My guy, you saved my brain cells from trying to understand how hybridisation and orbital overlap are related. Thank you
All the three sp2 hybrid orbitals are not identical. One sp2 hybrid orbital carry one electron while other two carries two electrons each?
Hey there, wondering why the oxygen MO diagram video is showing unhybridized orbitals while this video shows hybridized orbitals, thanks in advance for your help!
You have drawn singlet oxygen which is the first excited state. Normally oxygen will be in the ground state which is a triplet (di-radical) with no pi bond.
Why aren’t 1s orbitals depicted in the drawing? Are only the hybridized sp2 orbitals and 2p orbitals(needed for pi bonds) shown?
You can think of the sphere at the centre as 1s orbital, if you want to. Often the inside orbitals that do not interact with anything else is ignored while drawing diagrams.
@@Anandhu-X Thank you. Also, is the 1s orbital ever involved in hybridization?
@@thechemist6957 No, in the above mentioned sp2 hybridization, the s & p subshells belonging to principal quantum number 2 only (2s &2p) are involved.
@@thechemist6957 only the orbitals that are close in energies can mix together to form hybrid orbitals. that's why the lower energy 1s orbital is ignored as the energy difference is high because of one less electron shell.
why is there only 1 leftover p orbital? is it because there's only one pi bond?
How does oxygen end up with unpaired electrons in o2 then?
Actually the explanation is very good but the problem is that probably you are WRONG. I mean that in some places,I am seeing that they say that in such diatomic molecules, there is no hybridisation. In oxygen molecule, there will be simply a sigma bond between Pz orbitals of both atoms. I don't support them but please clarify.
i have this objection too
Yes exactly, i was thinking the same thing
YOU Saved my life thanks !!
Best explanation ever
That's art.. Beautiful..
I just wanna know where the lone pairs go
How many are from India fill attendance 👍👍
Lol only 24 from India 😂😂😂..
I guess Indians don't like to study
Thank you for the explanation
So O2 diamagnetic?
Thanks a lot for clearing my doubts😊😊
thank you king 👑
Very clear
Thanks sir
Powerful Sir
I mean here in Switzerland we learn the MO-Theory with O2 having only one bond in high school.
Love this
Very explanatory
aiyoo waltuh white yoo , wanna cook?
I love your videos , but the scratching of the sharpies are killing me soooo bad
Thank you 🙏 ❤
Thank you sir!!
Thank you
Good
Thanks sir
Thanks alot
Thank u can u make of these informative videos
2:41. 5:28