Titration of Strong Acid With Strong Base
HTML-код
- Опубликовано: 27 ноя 2024
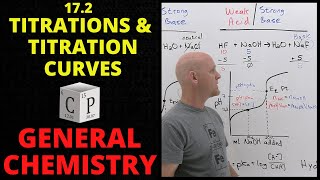
- One of the most commonly performed techniques in the general chemistry laboratory is the acid-base titration. This is an analytical technique that we can use to determine the concentration of some analyte by titrating with some titrant of known concentration. In this experiment we will determine the concentration of an HCl solution using NaOH as our titrant, and we will focus on the particulars of performing the titration rather than the accompanying calculations, which we have covered in other lecture-based tutorials.
Visit Thermo Fischer Scientific for all your laboratory needs: www.thermofish...
Follow Jamal Muhoza on social media: linktr.ee/dr_J...
Watch the whole Chemistry Laboratory Techniques playlist: bit.ly/ProfDave...
General Chemistry Tutorials: bit.ly/ProfDave...
Organic Chemistry Tutorials: bit.ly/ProfDave...
Biochemistry Tutorials: bit.ly/ProfDave...
Biology Tutorials: bit.ly/ProfDaveBio
Classical Physics Tutorials: bit.ly/ProfDave...
Modern Physics Tutorials: bit.ly/ProfDave...
Mathematics Tutorials: bit.ly/ProfDave...
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: amzn.to/2HtNpVH
Bookshop: bit.ly/39cKADM
Barnes and Noble: bit.ly/3pUjmrn
Book Depository: bit.ly/3aOVDlT








Thank you Professor Dave. This is like a dream coming true. Experimental techniques getting so handy.
Applying for a master degree in chemistry after a 5 years gap and the first degree wasn’t in English. I cannot tell you how happy I am to find your channel ❤️ This is very helpful. Thank you very very much
I work for ThermoFisher and Organic Chem Professor shares Professor Dave’s vids. This is awesome
I have to do acid titrations at work all the time to determine acid values of the various chemicals we manufacture. Great information!
I was taught (long ago!) that you never used a burette for alkali solutions:- the reason was that the atmospheric carbon dioxide would react with the titration causing a carbonate to form. Because this was insoluble, it then jammed the burette tap…
Titration with phenolphthalein must be carried out rapidly - again, atmospheric carbon dioxide (and that from the analyst) will cause the colour change!
You can eliminate the burette tip drop problem by using your wash bottle - just use a little squirt to rinse the tip of the burette into the test solution.
Just fyi, for accurate work you shouldn’t use sodium hydroxide solutions as a primary standard. Weighing out sodium hydroxide pellets isn’t accurate as they collect moisture from the atmosphere rapidly. You would take a primary standard eg oxalic acid which can be high purity, dry it at 105C, then weigh it accurately to prepare a standard solution. Then use that to calibrate your alkali…
And, yes, I’ve done that process many times!
Thanks for this playlist dr. Dave I will now proceed to produce controlled substances at an incredible rate
This was one of the best videos at explaining titrations
Back when I was 7 or 8, I saw my uncle determine the volume of an engine cylinder with a burette.
Things like that sparked my interest in chemistry, along with the shows on TV in the 60s!
Thanks Prof Dave
Morning peeps
plzz plzzzzz more of this!
Im in university studying chem eng and i find the labs pretty hard as i dont have much prior exp.
btw you rock prof dave
Exactly what I needed
I will always trust Prof. Dave with every cell in my body
No need for blind trust. Just learn things by consuming his contents.
Dave is HIM fr🥹🥹🥹🫵🏾
Thank you professor
I now understood the topic well
thermosfisher haunts my nightmares
I think its funny how this is more accurat and more deeply explained than my professor did when i tryed to lean this for my final exame
Will you be covering weak/strong acid/base titrations more in depth?
I remember this causing a lot of confusion for me and my peers in college.
Oh God. And then when you get an acid with multiple protons to donate... 😫😫
thanks!
Gas Chromatographs or Optical Spectrometry. Open one up and explain please.
Humans are Amazing btw.
Thank you once again for another amazing video!
Thank you Professor. Could you please make a video on redox titration ? Thank you
Thank youuuu 💐
Can it be explained exactly how phenolphtalein transform color from transparent to pink once the solution becomes basic? i.e. by what molecular process this phenomenon occur?
So once the equivalence point is reached proton transfer starts occurring with the indicator instead, and the two protonation states of the indicator interact very differently with light, so just transferring one proton makes it totally change color.
Yes, the color change of phenolphthalein from colorless to pink can be explained by the molecular process of protonation. Phenolphthalein is an indicator that exists in two different protonated states, a colorless, non-ionic form and a pink, ionic form. The indicator is colorless in acidic solutions, which means it is in its non-ionic form. In basic solutions, the indicator is protonated and it changes to its pink, ionic form. This protonation is caused by the transfer of a hydrogen ion (H+) from the surrounding solution to the phenolphthalein molecule. This changes the electron distribution in the molecule, which causes the absorption of light at a different wavelength, resulting in the change in color.
Additionally, it is important to note that this protonation process is reversible, meaning that if the solution becomes acidic again, phenolphthalein will lose the proton and will return to its colorless form.
Thanks 🙏🏻
Good
But what indicator are you using for this specific titration? It can't be the phenolphthalein you spoke of, because the equivalence point of that is at pH 8.3, and if a strong acid like HCl is titrated with a strong base like NaOH, then the equivalence point would be at 7.
Because pH is a logarithmic scale, the value changes extremely rapidly around the neutral point with only small changes in concentration. While you are technically correct that phenolphthalein will change state at a point past the absolute equivalence, the difference is small enough to be disregarded for the purposes of benchtop chemistry.
If you consider 10x more basic to be a small change, then sure.
@@jheadley635 Tell me you don't understand pH without telling me you don't understand pH.
@@sjholmesbrown Please professor, enlighten me. Shower me with your golden knowledge.
@@jheadley635 To change a solution from pH7 (equivalence point of this HClNaOH titration) to pH8, you only need to raise the OH- concentration to 10^-6M. If, as in this example, you're adding 0.1M NaOH, that means an over-addition of only 1 part in 10^5 is needed, or 0.001mL per 100mL of solution. If you can accurately dispense microlitre quantities from a titration burette, then please apply for a job with NIST, they'll be pleased to have you.
Great demonstration. I'm really enjoying this series. I wish I had this level of interest in chemistry back in high school!
Wrong usage of the burette.
After filling you let out a bit of the solution to make sure no air is left at the tap. Near the equivalent point you don’t add half a milliliter at a time; you do a drop at a time.
There was a drop left on the burette. That has to be added to the solution and then you will see on the color of phenolphthalein that it would go bright pink. You’ve added to much.
Not sure I would simply poor in alkali solution to be used after just having water in there. It should be flushed at least once otherwise you are potentially already diluting the known concentration before you even start.
So I've been wondering who is the gentleman running these experiments for you is this just some stock footage you found or it's just like a buddy of yours
A chemist I hired. I introduce him in the first video in this playlist! I also link to him in the descriptions.
@@ProfessorDaveExplains I missed that apparently. Either way very cool.
So concise and useful! How am I able to get this for free?
I thought Phenolphthalein was used for Strong base- weak acid titrations
How the hell do you do this with indicators that have barely any difference between the color it has at a pH < 7 and a pH of 7
David is a strong Hebrew name. I like it.
おはようございます
おはよう^_^
おはよございます!
おはようございます! uwu
Are you a spinner or a dripper?
I'm a spinner.
He's a dripper!
sir i am a very
I suck at titrations 😔
wDavE
Oh Lord, don’t you have titrant bottle already set up in this lab? What sort of outfit is this? 😂😂
professor dave thinks the world is round😂😂😂😂
Bait
Please explain, specifically, why you believe the opposite to be true, and then provide an experiment that can be performed to validate this.
If you wish, I am happy to provide any number of counter experiments demonstrating that the Earth is, in fact, round.
Yes you're "hiveminded"
What are you doing in a chemistry tutorial, sweetie?
@@ProfessorDaveExplains Most likely trolling and baiting replies
Donald trump makes sure everything is safe then he diffuses the stuff