Complex Ion Formation
HTML-код
- Опубликовано: 21 июл 2024
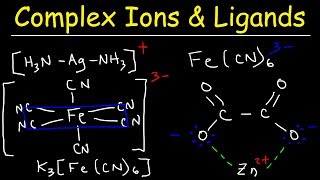
- Most transition metal cations can do something interesting in solution, they can interact with specific ligands to form complex ions. These coordinate covalent bonds are new territory, so let's get a mini introduction to inorganic chemistry right here!
Watch the whole General Chemistry playlist: bit.ly/ProfDaveGenChem
Organic Chemistry Tutorials: bit.ly/ProfDaveOrgChem
Biochemistry Tutorials: bit.ly/ProfDaveBiochem
Biology Tutorials: bit.ly/ProfDaveBio
Classical Physics Tutorials: bit.ly/ProfDavePhysics1
Modern Physics Tutorials: bit.ly/ProfDavePhysics2
Mathematics Tutorials: bit.ly/ProfDaveMaths
EMAIL► ProfessorDaveExplains@gmail.com
PATREON► / professordaveexplains
Check out "Is This Wi-Fi Organic?", my book on disarming pseudoscience!
Amazon: amzn.to/2HtNpVH
Bookshop: bit.ly/39cKADM
Barnes and Noble: bit.ly/3pUjmrn
Book Depository: bit.ly/3aOVDlT





![Jelly Roll - Dead End Road (From Twisters: The Album) [Official Music Video]](http://i.ytimg.com/vi/_Mb8CqKW4R8/mqdefault.jpg)


Thank you for your time and energy, Sir Dave.
keep up the good work professor. Hope you went deeper into the topic.
the way you presentate the video is excellent.
Professor have you make more videos on coordination compounds ?plz send links .
It is really helpful during pandemic
You are kind and you made chemistry so easy
Hi prof. Please can you me on how to prepare and analyze transition metal complex in titration format
professor what does form in water that is water-insoluble compound?
Professor, You must have 3 million + subscribers!! You're awesome... Thank you so much🙏
Yeah yeah I agree! Please tell your friends!
Almost there now
Thank you professor, you just made everything seem so easy. I wish you went a little more deeper into the topic.
yeah this one was a bit throwaway, i made it years ago for a company and the exclusivity clause ran out so i decided to post it, but i'll do more like it soon.
@@ProfessorDaveExplains thank you. Waiting for more:)
@@ProfessorDaveExplains thanks
P.dave thank so much I'm in high school your i like yoyr lessons because i studying like here in school you are my best science teacher allah keep you
can someone please tell me why it is 2 nh3
Thank you sir..need more vedios in coordination compounds..
i'm going to do a few on that this year!
Thanks prof!
Very useful
Please make a video on chelating reagent
thank you sir
Great sir
How is the overall charge of [Ag(NH3)2] positive? The silver is positive and the lone pairs on both nitrogen are covalent bonding? Shouldn't the lone pairs be -1 each?
NH3 is a neutral molecule, the lone pairs do not add extra charge. It has the same number of protons and electrons overall, so it is neutral.
Ag ion has +1 charge. So when you combine a +1 charged ion with 2 neutral molecules, you'd end up with +1 charged complex ion. Remember, charges must be balanced on each side of the equation.
Ag^+ + 2NH3 --> Ag(NH3)2 ^+
@@dr.oztekchemistrychannel9668 I don't understand why 2NH3? why not 4NH3 or any number? how to determine this number?
@@crieliocriel the number of ligands surrounding a metal ion in a complex is dependent on the SIZE of the central metal ion AND also the SIZE of the ligands around it. In this case, silver Ag+ ion is a very big ion (in second row of transition elements/d block or period 5). So it can ONLY fit two ligands around it forming a linear complex [Ag(NH3)2]+. Most complex ions can fit 6 or 4 (monodentate) ligands eg H20, NH3, Cl-, around their central metal ion. Silver is exception due to its large size.
Also When you add chloride ions to a solution of copper ions, there is a change in coordination number from 6 to 4 since Cl- ions are bigger than H20 molecules so can only fit 4Cl- ions around the central metal ion :
[Cu(H20)]2+ + 4Cl- = [CuCl4]2- + 6H20.
In this case, the change in shape of complex/coordination number is caused by change in SIZE of surrounding ligands in complex ion.
some guy looking like Jesus explaining the concept in 4:05 is better than 24+ hrs of reading textbooks
thank you Chemistry Jesus lol
Are the bonds ion-dipole within a complex?
nope they are coordinate covalent bonds
thx
I m so confused about are there complex compounds are not ions?
Do u have any Skype tutoring facility?
If u do, how much do u charge for it?
sorry i don't really tutor anymore!
No I'm just very busy with the channel and other projects.
So why do some ions have 4 ligands and some have 6 ligands? Is it to do with the size of the ligand?
yes and also the identity of the metal cation
Professor Dave Explains I see. Do transition metals behave similarly? Is copper unique in any way?
they all have their own properties!
Professor Dave Explains ok thank you
no it's not the size of the ligand
it's how you use it
Thanku sir
Good
I don't understand why 2NH3? why not 4NH3 or any number? how to determine this number?
correct me if i am wrong... if + the ligand will be 2.. if 2+ the ligand will be 4.. and so on??
@Khush Bakht Khan hahaha
i already got it though..
Professor Dave, You're awesome.
BTW, what are your future plans?
Just keep putting out as much content as I can! Also I have a book coming out.
@@ProfessorDaveExplains Oh wow!
which genre book?
Science-related or something else?
Social commentary about public science literacy! I'll make a formal announcement on the channel.
I guess i can't be an atheist anymore, my semester is being saved by Jesus itself
when Jesus himself comes to teach you chemistry
Is it just me or does he sound a bit weird at normal speed, maybe I'm just used to listening to him at 2x speed lol
thank you science jesus
i miss yu
jesus teaching chemistry
Quick correction: "AgCl is insoluble because lattice energy is greater than the energy of solvation" is a wrong statement. This fact only means the dissolution an endothermic process (ΔH>0). The spontaneity of the dissolution process (whether it will happen or not) depends on the free energy change (ΔG) of the process, which is equal to ΔH-TΔS, which also considers entropy. Entropy is part of the spontaneity discussion and must be considered. When both enthalpy and entropy are considered, it is seen that AgCl is insoluble (ΔG>0). Enthalpy alone cannot be used to discuss spontaneity.
Endothermic processes can be spontaneous, such as the dissolution of ammonium nitrate. For this process, lattice energy is greater than the energy of solvation, but the entropy term is a larger negative than the enthalpy term, making ΔG
but dissolution is always entropically favorable, so if something is insoluble, enthalpy must be the reason
@@ProfessorDaveExplains Hi Dave :) I find it necessary to add to the discussion that dissolution is not always entropically favorable. This may make an exothermic dissolution process nonspontaneous, if the TΔS term is larger than the ΔH term.For example compounds that contain ions with large magnitude of charge, such as aluminum chloride, have negative entropy of solution (-250 J/mol.K). The highly charged ions immobilize water molecules more than ions with low charges would. So although the ionic compound experiences an increase in entropy, the solvent experiences a larger decrease in entropy, and the overall process will have ΔS
interesting i was not aware!
ok sure
kk
hello my name is Aaayons
I live in AAYON
😂 😂 😂
Cool....
Am the first comment
Give this guy a Nobel prize
@@tyrekeholness1687 hey really???
Bolna toh sikhle kiya bol raha hain
can you be a little bit more bored when explaining? 😅
this made no sense to me
Thank you professor but i still couldn't understand even a single bit of it. Useless video... found a replacement for it from an Indian channel
What's the Indian channel
Why the hate?