Acidic Basic and Neutral Salts - Compounds
HTML-код
- Опубликовано: 25 июн 2024
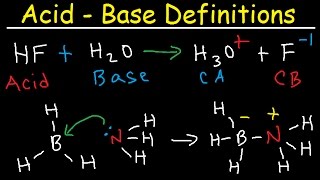
- This chemistry video tutorial shows you how to identify an ionic compound as acidic, basic, or a neutral salt. You need to know the 6 common strong acids such as HCl, HBr, HI, HNO3, H2SO4, and HClO4. The conjugate base of a strong acid is a neutral anion. The conjugate base of a weak acid is a basic anion. This video contains plenty of examples and practice problems. Here is a list of compounds included in this video: KCl, NaOH, NH4Cl, AlBr3, NaF, FeCl3, KH, KI, LiNO3, KNO2, LiC2H3O2, CuSO4, K3PO4, LiI, NH4NO3, CaO, AlCl3, NaClO, Na2CO3, Ba(OH)2, Na2SO4, K2SO3.
Acids and Bases - Introduction:
• Acids and Bases - Basi...
The 7 Strong Acids to Memorize:
• How To Memorize The St...
Conjugate Acid-Base Pairs:
• Conjugate Acid Base Pa...
pH and pOH Calculations:
• pH, pOH, H3O+, OH-, Kw...
Estimate The pH Without a Calculator:
• How To Calculate The p...
_______________________________
Autoionization of Water - Kw:
• AutoIonization of Wate...
Which Acid Is Stronger?
• Acid Base Strength - W...
Acidic, Basic, & Neutral Salts:
• Acidic, Basic, and Neu...
pH of Weak Acids:
• pH of Weak Acids and B...
Buffer Solutions:
• Buffer Solutions
_________________________________
Polyprotic Acid Base Equilibria:
• Polyprotic Acid Base E...
Acid Base Titration Curves:
• Acid Base Titration Cu...
Acids and Bases - Practice Test:
• Acids and Bases Review...
Ksp - Molar Solubility & Ice Tables:
• Ksp - Molar Solubility...
Complex Ion Equilibria:
• Complex Ion Equilibria...
___________________________________
Gibbs Free Energy, Entropy & Enthalpy:
• Gibbs Free Energy - En...
Entropy - 2nd Law of Thermodynamics:
• Entropy - 2nd Law of T...
Electrochemistry Practice Problems:
• Electrochemistry Pract...
Final Exams and Video Playlists:
www.video-tutor.net/
Full-Length Videos and Worksheets:
/ collections
Chemistry PDF Worksheets:
www.video-tutor.net/chemistry...








Chemistry PDF Worksheets: www.video-tutor.net/chemistry-basic-introduction.html
Final Exams and Video Playlists: www.video-tutor.net/
hey dad
looking back at this video after my test, I've found a much better way of explaining it. Group 1+2 elements are negligible (neutral) because they COULD have come from a strong base (OH). For strong acids, you just have to memorize them. You have to remember, when a compound is not a strong acid, it's essentially a weak acid. You can also consider cations/anions when determining acidity. Cations (positive charge) = acidic and anions (negative charge) = basic
THANK YOU. I knew there was a better way to do this. This guy is good but he fails to point out key factors at times...
🙏
I kept having problems with this because there are some compounds that at first look like it will be acidic but then its basic. Great tips you came up with, will jot them down. :)
Thank you!!!
I literally was thinking the same thing as well. This comment literally brought me some sureness in what i wrote in my notes
Posted this is someone's comment and it helped them so I hope it helps you!
If you write H-OH underneath the compound then cross them youll get two new compounds. If its a strong acid + strong base the salt solution is neutral. Strong acid weak base = acidic and strong base + weak acid = basic.
Ex: NaClO would produce NaOH + HClO. the answer is basic because the NaOH is the strong base and wins over the weak acid. Hope that helps.
Thank you good sir!!
Would not work for NH4NO3. Only works for some salte
thank you so very much
@@rileyj.s.5899 yes, it would still work, just slightly different. NH4 is the conjugate acid of the weak base ammonia, so its a relatively strong acid. nitrate ion is the conjugate base of nitric acid, so ammonium nitrate would be an acidic salt. NH4--->NH3 NO3--->HNO3 : HNO3>NH3 therefore acidic. The first way is confusing but correct, the second is less accurate if you think about whats physically happening, but is easier to grasp and apply.
Meetal Patel that helps a lot thanks 🙏🏼
Think of it like this:
1)Anything that’s in group 1 or 2 can be cancelled out of the equation. (Neutral)
2) anything that was made from a strong acid such as cl- from HCl can be cancelled out (neutral)
3) if it has a positive charge it will make a acidic solution
4) if it has a negative charge it will bond to h+ and reduce the h+ concentration in solution and therefore make it more basic.
(Mostly made from weak acid conjugate).
If anyone is wondering. Al+3 and all others under the Acid category are consider acids because they are attracted to electrons, thus electrophile or lewis acids. Lewis acids are electron acceptors. Remember this.
LEWIS ACCEPTORS
Amphiprotics
I love chemistry subject but I am getting confusion
Vishal S
really
I really appreciate this comment because as of right now, I too am getting confusion
@@ubebabe. meee nowww :D
Am playing through FE3H and this comment just gave me HEAVY Petra vibes.
Just remember the *strong acids* (H2SO4, HI, HBr, HNO3, HCl, HClO4) and the *strong bases* (LiOH, NaOH, KOH, Ca(OH)2, Sr(OH)2, Ba(OH)2) and *their ions will always be neutral*
The rest of the ions containing *positive charge will be acidic* (Lewis acids are electron acceptors).
Similarly, the ions containing *negative charge will be bases*
You can use the following *mnemonics* to memorize the strong acids and bases respectively:
• SO I BRought NO CLean CLOthes.
• Lets Not Keep Causing Strong Base Reactions
These align to the order given above.
My way for it which is essentially similar is, you take the compound and you split it into anion and cation, then you put H with the anion and OH with the cation, like if he gave you
NaCl, ----> NaOH + HCl
And then you memorize strong acids and strong bases, if the two compounds you get are a strong acid and a strong base, this is neutral
If the two compounds you get are a strong acid and a weak base, it's an acid, if it's a strong base and weak acid, it's a base. If both are weak it's also neutal
Thanks, this helped a lot😊
@@euthopianeutho8742 wow i am glad, any time bro
after going through many videos I realized the best thing to do is to just memorize the table he talked about.
well if you know your strong acids and bases you dont have to memorize the table and just realize if its an CB or CA of a strong base or acid its negligible and all other cations are acidic except t-metal +1 and all anions are basic except conjugates of SA etc.
Thanks to the comment section and Organic Chemistry Tutor, I now understand this thing and am ready for the test!! Lets get that A!!
This is the best video to know about acidic,basic and neutral solutions.
I realised that you are the only youtube who does not say like, share and subscribe in the end
you only say that have a nice day
that is what makes you special
are the elements/compounds you listed the only ones that are neutral/basic/acidic? or are there more? im referring to the table that you made at 4:22
The chart helped especially. Thanks for a clear explanation!!
This is very good! just ignore those people with negative comments.You are helping people like me a lot! keep up the good offer!
You should teach my Chemistry class instead of my current teacher! Thanks a lot professor!
He's a tutor because professors do not have the time to go in to depth. There is a huge pedagogical difference between teaching and tutoring.
👏This is actually one of my favourite videos
Chemistry is not really my thing, I feel I am forced to learn it and this video really did help me, thank you
A new learning
reactions very good
OMG thank you! 🙏🏻
Thank you, this helped alot
Stop sir, your teaching is really good💞 much love and thank you for clearing the doubt I had.
great video!! you always help a lot, ignore the negative comments, no idea how any of this is confusing to people, you explained it very well!!
chem mock tmr (paper 4) and im cooked dont know shi but hope this random guy saves me
Thank you friend 👌👌👌🖒🖒🖒
00:02 Identifying acidic, basic, or neutral salts based on acid strength
01:27 Conjugate base of strong acid is neutral.
02:40 Salts can be acidic, basic, or neutral in aqueous solution.
04:07 Determining if compounds are acidic, basic or neutral
06:09 Different salts can be acidic, basic, or neutral based on their chemical properties.
07:12 Different salts can have acidic, basic, or neutral properties based on their constituent ions.
09:24 Sulfuric acid has properties between neutral and basic, classified as N/B salt.
10:30 Acidic and basic compounds ionize water to release H+ or OH- ions in solution
I love this video really very helpful to me thanks a lot
😊
strong acid +strong base =neutral
strong acid+weak base =acidic
weak acid+strong base=basic
think of it like the salt type will resemble the stronger reactant
and for the other part the conjugate acid of a weak base is strong acid and the conjugate acid of a strong base is weak acid
and the same for acids too
AWESOME !!!
I kinda wish that it would be explained in more detail, BUT it still helped a lot
he teaches biology too
Thank you!
Thank you!!
On Point!
you really helped me buddy
THANK YOU MAN
I have a question elements having a positive charge have tendency to lose electrons and are acids and those who attract electrons gaining a negative charge is a base , is this true?
Good work
Thanks alot!
Thank you very much
wow thank you for this
Thank you ✨
Great Video; However, I have a question---> you mentioned at --10:17-- that F- is a weak base.
How so when it's from HF which is a weak acid? or is it related to the high electronegativity or instability due to tight electron cloud for Fluorine anion?
I am confused :3
Acids and bases are little bit confusing but there are tackling techniques my Professor gave me.
Just memorize these as your strong acids: HI, HBr, HCl, H2SO4, HNO3, HClO3, HClO4 then when you are given any acid that is not one of this list just know it is a week acid.
weak acid-partially ionizes in aqueous solution.
strong acids- completely dissociate or ionizes in aqueous solution.
Ty, and hope u have good day too =)
Is there any Acid salt whose aqueous solution is basic? I saw in the exam paper of MEXT? off course there are choices to select?
Thx you!
So if a salt is considered an acid and a base, what are two neutral ions, such as KCl, considered?
Easily understand in basic Acid and structure with formulas pls kindly share the Ideas
But how do u know if something is acidic neural or basic of looking at it or do u have to just memorise them
So would alcohols and sugars be considered neutral since they don't usually ionize therefore they don't affect the pH
Thank you
Can u drop a video on salts, types, preparation and properties
for any aluminum polish compound, sir, the name of the material and its preparation, for polish compound step 1 and step finish, thank you
Thank you so much!
Does anyone know of any material to help prepare for acs? for basic chemistry?
Strong Acid conjugate bases, and strong base conjugate acids, seem identical to the soluble ions from the Solubility Chart for determining if aqueous reactions occur
that's because all of this is related to intermolecular forces, but nobody ever mentions that.
Can i know which application is used to write to make this video?
you are amazing
Was absolutely helpful! Thank you!
Thanks
thanks you chemistry it is a good subject
I understood finalllllllly God blesses u hugely
finally got this down
I love Chemistry and I hate my teacher..but I will show him how I'm excellent
Anwaar alramidhi
anwaar alramidhi
What about-Ca(H2PO4)2...
Acidic or basic?
Please explain how the HOCl is acidic?🙏🙏
I'm not entirely sure, but I think its because if you react it with water, it will act as an acid by donating its H to the water to make H3O.
Didn't you read it the other way around? I believe it is "HClO"?
THANK YOU BUT PLEASE CA?N YOU ANSWER ME IS CH3OH2OH ACIDIC OR BASIC SOLUTION
what about hclo3 thats a strong acid?
why is hno2 a acid if no2 - and h- are both basic?
This topic is chemicals nature of acidic basic or neutral me wrong or Correct please 🙏 help me
thank u so much. just learned this in class and was confused. now im not :)
I'm glad to hear this was helpful to you.
🎉 thank
the halide ion is extremely kya hoga
at 9:20 ish, isn't HClO a strong acid, so NaClO would be neutral??
No, I think you're getting it confused with HCl. HCl is a strong acid, HClO is a weak acid. Since it's a weak acid, NaClO is a basic solution.
The stronger the acid, the weaker the conjugate base.. and vice versa (because Ka x Kb = Kw, a constant). HClO4 is a strong acid... (Note: some sources also consider HClO3 to be a strong acid)... so its conjugate base, ClO4-, would have negligible strength.. thus a solution of NaClO4 (which fully ionizes into Na+ and ClO4-) would be neutral. However, HClO is a weak acid, thus its conjugate base, ClO-, would also have relatively weak strength. So NaClO (which fully ionizes into Na+ and ClO-) would be slightly basic.
Could you also say that because HF is a weak acid, we know its conjugate will be stronger?
Yes it’s conjugate will be a strong BASE
What is BaF2
Neutral is NaCl too
Chemistry to confused students:
Hamare yaha Aisa he hota hai😂😂😆😂😅😂
Sir how can we identify the substance is acidic or basis
It will dissociate completely
Can somebody tell me why he'd written H-? isn't it H+?
I'm confused at KH. Everyone I go I find that H+ is said to be an acid, yet you say it's a strong base
This isn’t H+, it’s H-, hydride. Hydride is a strong base.
3.63 MILLION SUBS WHWATTTT
Mostly imp. 5:20 to. 7:30. Second
I think the video miss several points.
What is the purpose of the video:
to teach you how to determine if salt(compound form) is acidic, basic, or neutral based on its composition ion
pt1: introduces us which ones are strong acids(memorize) and weak acids(memorize)
pt2: -> understand that acid* generate conjugates bases, [base generate conjugate acids]
strong acids* generates really weak conjugate bases(so weak that we consider them neutral)
weak acids* generates decent strength conjugate base.
note that the acid with a *denotes the compound form and conjugate acid is in ion form:
ex:
weak acid: HNO2 has a decent conjugate base: NO2-
strong acid: HNO3 has a weak/neutral base: NO3-
pt3: understanding that we can use this conclusion from pt2 to see if an ion is neutral or basic:
ex: NO2- is a basic ion, NO3- is a neutral ion
pt4: other ions have different properties:
ex: K+ ion is neutral b/c it is alkaline
pt5(key):
combine all the characteristics to determine if the salt compound is acidic/basic/neutral when dissolved in water, because when it dissolves in water, it is seperated into its constituting ions, and therefore we can use the acid/basic/neutral property of the ion to determine the same properties for salt.
ex:
KN02=??
As I said before:
pt4: K+ ion is neutral b/c it is alkaline
& pt3: NO2- is a basic ion because pt2: weak acid: HNO2 has a decent conjugate base: NO2-
neutral+basic=basic
so KNO2 is basic.
Doing gods work
Na2co3 strong or weak????
weak
Fe is acidic O is basic then why FeO is basic?...plz reply
O is a strong base and Fe is a weak acid so the O will "overpower" the Fe
🎉🎉
Is KI acidic or basic?
Neutral.
I do not get it
What about HSO4-
HSO4- is the conjugate base of the strong acid H2SO4 (sulfuric acid). However...H2SO4 is polyprotic, meaning HSO4- is, despite being a conjugate base, a weak acid. When finding the pH of a salt whose anion is hydrogen sulfate, treat it as a weak acid.
What's your name?
julio
Hi
NH4Cl . NH4 is a weak acid so the solution is acidic
LiC2H3O2 . C2H3O2 is a weak acid so the solution is basic
Can you please explain what the difference is
Thank you
sorry buddy ):
I think it has to do with their charges. NH4 has a positive charge, and it's not one of the strong acids, so it must be acidic. C2H3O2 has a negative charge and it's not a strong acid either, so it must be basic. Another way to remember this would be to think of the Bronsted-Lowey definitions of an acid and base. A Bronsted-Lowey acid donates proton H+ while a Bronsted-Lowey base accepts proton H+. So if it has a positive charge, it is willing to give away a proton H+ which makes it acidic. If it has a negative charge it will accept the proton H+ which makes it basic. I hope this helps! Also, thank you for asking the question! I didn't realize that until you brought it up, so I was trying to figure out the answer myself. This helped me understand this topic even more. Thanks!
Hi
what?
Phosphate salts: foe's fate (salt)
h
I still can't understand 😥
tomorrow is my exam
🤓
I miss STEM schools
What is the rules?? This is not helpful at all
If you write H-OH underneath the compound then cross them youll get two new compounds. If its a strong acid + strong base the salt solution is neutral. Strong acid weak base = acidic and strong base + weak acid = basic.
Ex: NaClO would produce NaOH + HClO. the answer is basic because the NaOH is the strong base and wins over the weak acid. Hope that helps.
maya patel thank you so much 😊
That’s awesome 👏
@@salwaramzy9985 My pleasure! =D