Solubility Rules
HTML-код
- Опубликовано: 10 окт 2024
- This chemistry video tutorial explains how to use the solubility rules to determine if a compound is soluble or insoluble.
Stoichiometry Practice Test: • How To Solve Stoichiom...
Solute, Solvent, & Solution:
• Solute, Solvent, & Sol...
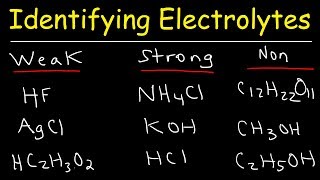
Strong & Weak Electrolytes:
• Identifying Strong Ele...
Molarity Practice Problems:
• Molarity Practice Prob...
Ion Concentration In Solutions:
• Ion Concentration in S...
Dilution Problems:
• Dilution Problems, Che...
___________________________________
Types of Chemical Reactions:
• Types of Chemical Reac...
Solubility Rules:
• Solubility Rules
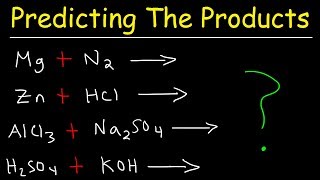
Predicting The Products of Reactions:
• Predicting The Product...
Activity Series of Metals:
• Activity Series of Met...
Will This Reaction Occur?
• Chemistry - Will The R...
Predicting Products of SR Reactions:
• Predicting Products of...
___________________________________
Double Replacement Reactions:
• Introduction to Double...
Net Ionic Equations:
• Precipitation Reaction...
Writing Chemical Equations From Words:
• How To Write Chemical ...
Solution Stoichiometry:
• Solution Stoichiometry...
Molarity & Dilution Problems:
• Molarity Dilution Prob...
Acid Base Neutralization Reactions:
• Acid Base Neutralizati...
____________________________________
Acid Base Titration Problems:
• Acid Base Titration Pr...
Mixture Problems:
• Mixture Problems
Calculating Oxidation Numbers:
• How To Calculate Oxida...
Oxidation and Reduction Reactions:
• Oxidation and Reductio...
Balancing Redox Reactions:
• Half Reaction Method, ...
Ideal Gas Law Problems:
• Ideal Gas Law Practice...
___________________________________
Final Exams and Video Playlists:
www.video-tuto...
Full-Length Videos and Worksheets:
/ collections


![VENEZUELA vs. ARGENTINA [1-1] | RESUMEN | ELIMINATORIAS SUDAMERICANAS | FECHA 9](http://i.ytimg.com/vi/SJfoPdeOPCQ/mqdefault.jpg)




Final Exams and Video Playlists: www.video-tutor.net/
i’m gonna cry
real
Real
Real
Real
real
Thank you so much! You really brought my grade up for my Calc AB, Calc BC ,and Chemistry! Your teaching methods are truly amazing :)
Perfect timing in studying for the MCAT!
Good luck!
i'm studying for the NMAT (mcat in the philippines) right now. his videos are a great help. good luck on your exam!
Good luck! 💜
What’d u get,
How did you do? 😅
Thank you, very helpful!
I would like to point out there are exceptions for S^2- ion. Exceptions include group 1, ammonium, and Ca^2+, Sr^2+, Ba^2+ if your instructor is going by those exceptions. So I got CaS is soluble and aqueous!
Other than that, very helpful explanations and practice!
i thought i was getting more confused when he said CaS is insoluble, now i see i'm on a right track
Words will be less to tell u how much u are helping me...u are a blessing. U explain everything with so much clarity and simplify take a lots of lots of lots of love from Bangladesh 🇧🇩 💖💕
I really enjoyed watching this video because it goes into depth about how to know which compounds are soluble and insoluble according to the solubility rules, which is important to know for chemistry experiments. One way to know if a compound is soluble is by looking at your periodic table and seeing which column the compound is placed, generally.
What can I say except thank you , literally no words for you ❤
Very very very beautiful and important topic of inorganic chemistry..... Thank you so much
Wouldn’t CaS be aqueous? (5:48) because sulfides are insoluble but it has an exception of Ca.
Felt that
The exceptions are for each row. The row with S doesnt have Ca as an exception
Ca+ is an exception for SO4 not sulfide. The exceptions for sulfides are group one metals and amonium.
@@lgnobil no, sulfides of group 2 cations and hydroxides of Ca, Sr, and Ba, are slightly soluble.
Perfect timing for this summer class haha
This man can teach better then my chem teacher
Good job
Professor Organic Chemistry Tutor, thank you for a detail explanation of how to use the Solubility rules to determine if a Chemical Compound is Soluble or Insoluble in AP/General Chemistry. Any compound that's dissolve in water is soluble, otherwise they are Insoluble. This is an error free video/lecture on RUclips TV with the Organic Chemistry Tutor.
gonna ace this test thanks bro
Metal Oxides *insoluble* except Group 1 + Castro Bear
Hydroxides *insoluble* excpet Group 1 + Castro Bear
Sulfate salts *soluble* except Castro Bear
And what’s Castro Bear again
@@Vunguyen-zd5jr Ca Sr Ba
i just want you to know that you save lives every day
Your videos are REALLY helpful. 👍 is there anyway that you could deliver a few voluntary classes online to a group of students? I'm Dr Quratulain Bukhari, my husband and I take voluntary classes and also conduct voluntary programs for the children of our community. We would be glad if you could take a few classes on the core Chemistry concepts. Please do let me know.
Makes it easier to understand
Thank you for the clarity sir
I swear you're gonna save my ass next term seeing as how I failed this term.
I love you so much
At one point he accidentally says “sodium chloride will form a solid” in water. He meant to say “silver chloride”
you just gained a subscriber
I love you so much
Midterms is this Monday
I would like to add the fact that Tellerium oxide is a strong base and dissolves in watter compleately
THANK YOU
You're fabulous
Thank you sir
Thanks got my finals in 30 minutes
bro me too
Thank you
Ty
Thank you, sir
The best
This is gona help my history💦
What is the solubility ?
What if there were transition metals in the compounds would these rules still matter?
Watching this a month before my exams😭😭
Na2CrO4 (s or aq)??
aq
Isn't CaS soluble because its an exception?
Isn’t CaS soluble? It’s an exception to the sulfide ion rule
calcium is not group 1 its a group 2 metal
i love you
Anyone here from ap chem
Is cobalt di iodide solid or aqueous?
slayed
isn’t OH soluble with K?
NICE
i love u
4:28
Midterm in one hour, I might be cooked
I AM JUST A HUMAN AFTER ALL😢
I feel like death
SUPER LOUD AUDIO!!!!
ca(oh)2 is not soluble in water
Crying
First one here doesn’t this deserve a like from you guys? 🧐🤣
No
nicole peterson 🤣
3:27 wut
What if there were transition metals in the compounds would these rules still matter?