Thermodynamics - 3-5 Pure substances property tables - Changing states example 1
HTML-код
- Опубликовано: 13 окт 2024
- Download these fill-in-the-blank notes here: drive.google.c...
Pure Substances
Water and Refrigerant
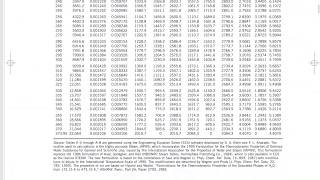
Thermodynamics Property Tables
Saturated liquid vapor mixture. Quality. Specific Enthalpy.
Rigid tanks. Closed Systems
And now you can purchase my Thermodynamics notes completely filled in (184 pages of notes and problems) for $50 here: sowl.co/ksG5e








Your students are very lucky. When I was in the university, we had to deal with much harder problems in the thermodynamics exams, including double interpolations and complex systems.
By the way, your lectures are excellent, too.
Hangi üniversitedeydiniz hocam
Hey. shouldn't we also be using 0.0014 for v in the first part?
Yes, it looks like I accidentally used the volume of 0.014, but should have been using the specific volume of 0.0014.
But I think all the other numbers (the x and everything else) are still correct. I just miswrote it wrong there.
@@engineeringdeciphered oh okay👌🏽
@@engineeringdeciphered came to comments to also as that thanks sir!!
I think the Volume is 0.014 m^3 , which means the 14L/1000m^3 = 0.014m^3
the quality X will be 0.19 for state1 and 0.393 for state 2 .. Am I right?
I think the same; because in state 2 (vf & vg) changed, so the quality (x) must change to be equal to 0.394😅
And H = 1527.846 kJ
specific volume was used, meaning 0.0014m^3/kg.
This is a great playlist!! Do u have one for thermo 2 as well? i m taking it next semester after this course.
I think in state2 x isn't the same value as in state1; because in state 2 (vf & vg) changed, so the quality (x) must change to be equal to 0.394😅
And H = 1527.846 kJ, am I wrong teacher?
Professor, how did you find T= 0.61 C? Thank you in advance!
I got it... through interpolation of the Tsat between 280 and 320 Kpa.
@@mothug978 can you explain it please
@@abdallahhamdan4505 Your pressure = 300 Kpa, and need to find Tsat. By using Table A-12, you have to interpolate between pressure 280 Kpa and 320 Kpa to find your Tsat. Hope that helps
How would I know if the 'x' value makes sense or not?
X is zero if it’s a sat liquid and x is 1 if it’s a sat vapor. And x = 0.5 if it’s halfway between. So if your value is closer to the sat vapor value then your x should be above 0.5. If your value is very close to the sat vapor value then your x should be closer to 1.
@@engineeringdeciphered Thank you very much
how do u find t
why didnt the equation for X, mass of vapor over total mass work ?
Because we don’t know the mass of the vapor. So the way I did it is easiest. There is probably a way to find the mass of the vapor but it would be a lot more work than the way I did it.
@@engineeringdeciphered Thank you.
Professor where's the question 10 .Before this video its Q9 and in this video Q11
Can I get that table?
www.dropbox.com/s/tuqy5e8657ysoda/Property%20Tables%20-%20Appendices%201%20and%202.pdf?dl=0
You can also find the tables in the internet.
x should equal to .19746
for the first problem
@@walkingunknown07 did you see this other comment? I think I miswrote .014 and should have been .0014. So I think my final answer is the correct one?
i think you made a mistake calculating vg