Combined Gas Law || Chemistry with Dr. G
HTML-код
- Опубликовано: 3 янв 2025
- Gas behavior can be defined by various physical properties, such as the pressure, volume, temperature and amount (# moles). The combined gas law expresses the dependence of temperature, pressure and volume for a fixed amount (# moles) of a gas.
The combined gas law combines Boyle's Law (P-V), Charles' Law (V-T), and Gay-Lussac's Law (P-T) into 1 relationship.
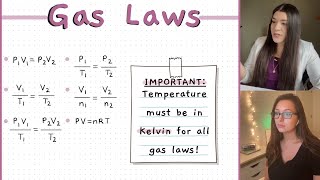
P1V1/T1 = P2V2/T2, where P1, T1 and V1 represent the Pressure, Temperature and volume in 1 condition of the gas and P2, T2 and V2 represent the Pressure, Temperature and volume in another condition.
![Master the Ideal Gas Law in Chemistry - A Step-by-Step Guide - [1-5-10]](http://i.ytimg.com/vi/kZIpV4RcVZ0/mqdefault.jpg)
![Master the Ideal Gas Law in Chemistry - A Step-by-Step Guide - [1-5-10]](/img/tr.png)