An Introduction to Chemical Kinetics
HTML-код
- Опубликовано: 23 июл 2024
- In this video I introduce chemical kinetics and it's relationship to reaction rates and mechanisms. We discuss the factors that affect reaction rate and how stoichiometry affects relative rates.
Chemistry lectures and "How to" videos from a university professor and Ph.D. chemist. I live stream office hours every week and discuss the latest news in climate change, space travel, and the intersection of science and politics. Come join the Melko Lab and learn by osmosis!








Amongst all the RUclipsr i know, you are the one who made me understand this very well...thanks bro!
This was so helpful!!
ti was really helpful thank you :")
Thank you can you give us this pdf?
Thank you 😊
Why did we multiply everything by 3 to find the final answer in the last practice problem
thank you sooo much!
i love it thanks so much !
Was helpful
Thanks
nice explanation
Easy to understand
Thanks you 🥰
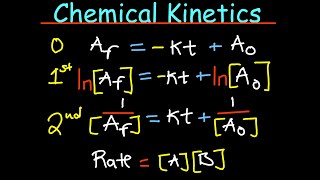
Bookmark 15:00 plotting rate data
Thanks I appreciate
thank you
Thanks 👍❤️
wonder if i’ll learn this someday
take engineering and its in 1st semester chemistry :(
Hi, in the last problem of the video, in the equation i might consider rate of the hydrogen, example: -d[o2]/ dt - 1/2 d[h2]/dt = 1/2 d[h2O]/dt? or not?
Yes you may write an expression in terms of H2:
-1/2 d[H2]/dt = 1/2 d[H2O]/dt
However, since the problem is providing you a rate for O2, it makes more sense to write the rate expression in terms of O2 and go from there.
In a bimolecular elementary Reaction
When the concentration of one of the reactants is in large excess compared to the other, the rate of the reaction becomes independent of the concentration of that reactant.
Is this statement true or false,could you explain
Yes this is basically true. Imagine you are making sandwiches and that you have 1,000 slices of bread spread on the counter but only 10 slices of cheese somewhere on the counter. The rate at which you can make sandwiches is really only determined by your ability to find the cheese, because there is bread readily available everywhere (it is in large excess).
God bless u
22:20 why is it not -0.250M/min?
if delta [A] is -0.750, then isn't delta [B] just -0.750/3 ?
from what I understood, u need to cancel the three from the right equation, in doing so u need to multiply 3 on both sides, that is why -0.750x3=-2.25 idk if this would help
@@aishahplaza808 i mean actually it's 3/0.750 equals to 2.25💀
@@yeshasmarak6365 i mean use calculator bruh
@@aishahplaza808 yeah yeah I'm sorry i didn't see that is was 0.25 💀 (i also forgot to correct the comment too.)
❤️
Good
so many lightbulb moments I've had in this one video lol
We can see the full screen....l didn't see the examples
can i invent a thing through chemistry can stop the mechanism of converting food to fat into body and instead maybe converting to muscle or just take the nutrients and throw the rest to poop cuz i want to stop getting fats on my body :)
Sadly no. Maybe in 100 years we'd be closer to that, but no time soon :(
0.750 / 3 =0.250