Molecular orbitals - HOMO and LUMO in easy way
HTML-код
- Опубликовано: 25 июл 2024
- What is HOMO and LUMO ins spectroscopy? HOMO is the highest occupied molecular orbital whereas LUMO is the lowest Unoccupied molecular orbital. Here in this video, we will see how they are formed in the molecules.
#homo
#lumo
#frontiermolecularorbital


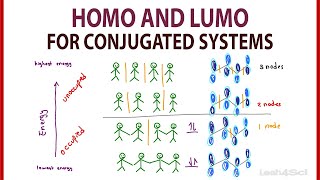






The concept is so perfectly explained, helped a lot , thank you so much ❤️
Glad it helped! Thanks for watching.
I always understand whatever you explained in your videos. Always very well explained and illustrations are on point. I find it easy to follow you while explaining and hats off! Thank you for the time and work you put in your videos! You are a life saviour!!
@Amit Choudhary why p orbital has two different colours that is dark blue and sky blue 😵🤔🤔
Very useful one 🙏🏻 ..... indeed.
@@ashutoshbhensadadia451 please reply my comment also
I really loved the explaination. Now my concept is clear.
orbitaaaal ....wow🤣👌
btw thanks a ton for.... covering everything in precise way
people like you just focus on the mistakes
I just turned on closed captions and turned off the sound. I'm a very visual learner, so it's hard for me to follow along with any narration. Is anyone else here cram studying for a test? I sure am 😅
@@amit_jaat44 why p orbital has two different colours that is dark blue and sky blue 😵🤔🤔
HOMO/LUMO is so important that can simplify literally all the reactions, which sounds a little overexagerated but it appears to be true. Regioselectivity or stereoselectivity are common examples that how they works, and electronic theory often fails to.
Excellent Explanation Sir!! keep Going❤️
Well explained, great video
Helped a lot
I liked your slang very much than the concept 😁
Shame on you
@@micronano9510?? Why to be shame
Yyiii
Very well explained! Thank you!
you dumbed this down perfectly for my little brain, appreciate it
Simply excellent bro
Clean clarification!!!!!!!!!
Sir I highly appreciate your videos it brings back my confidence which I had during neet UG preparation as my all concepts are cleared from your videos.
That I C/N do in my Pharmacy College.
Glad to hear that. Thanks for watching.
True boi ❤️
Tremendous teaching style very useful video and very thanks to you🙏
why p orbital has two different colours that is dark blue and sky blue 😵🤔🤔
Hi sir can u make more and more videos on medicinal chemistry
Excellent illustration and explanation.. thank you
@Amit Choudhary why p orbital has two different colours that is dark blue and sky blue 😵🤔🤔
You're a great teacher
wonderful explaination
very great explanation
Can you show exactly how the jumping of electron takes place and how many electron and from which orbital
Thank you sir 🙏🙏🙏
Sir very easily understood
Thanks a lot.
Great..thankyou
Cant believe i really understand it thank you sirr
@Amit Choudhary why p orbital has two different colours that is dark blue and sky blue 😵🤔🤔
Excellent explain
Thank you sooo much 🌺🌺🌺
Thank you!
Thank you sir.
Thank you very much sir..
Good explanation. Thank you
You are welcome!
i had to use subtiltes but thx a lot for this vid. i will watch it 2-3 times again to undestand the topic
Changed my life
Thank you 😊 sir
Excellent tutorial video.plz make more videos based on molecular orbital theory!!
@Amit Choudhary why p orbital has two different colours that is dark blue and sky blue 😵🤔🤔
@@sanjaymahaseth3764 different phase man
@@sanjaymahaseth3764 u need to study basics of quantum chemistry then u will understand.
@@vulgarteachWhere will I get in chapter 2 or 3 of class 11th chemistry.
The chapter 2=Atomic structure
Chapter 3=chemical bonding
@@sanjaymahaseth3764 u won't find it in 11th that's way too basic u may find in standard books like Atkins and Puri Sharma
love it
Well explained ❤
excellent explanation
Superb explaination !
Glad you liked it. Thanks for watching.
Best explanation
WOW !
KING !!!!
But why you have told homo is more energetic than lumo,,,in your lecture,,,, so, I'm confused about,,, make it clear, in comment plz
Thanks v v helpful
Make a video on NMR numericals solving only by NMR data plzzzz
Sure
👍👍👍👍 wow sir
Is there video on R-Li homo
Thanks Sir 😊
Thank K you ❤😊
Welcome
Very nice
😍😄😍😍
Can anyone pls clarify my doubt ?
How can atomic orbitals overlap both in phase and out phase simultaneously ..i mean how can 2 atomic orbitals interfere constructively and destructively at a same time ?
Some one pls help me ,im tired of thinking about this but i cant stop thinking abt this .
I mean its just that think of 1 orbital as 1 wave and other as another wave if they super impose then they form a molecular orbital if they undergo destructive interference then they form abmo.
Here +ve phase overlaps on +ve phase of adjacent atom and same goes for -ve phase making a amplitude larger(constructive interference)
Amazing exppaination
Thank you !
exppaination means?
waw thats crazy now i wony fail oc
Me - hybridisation
My teacher = hyyybriiidiiisaaatttiiiioooonnnnnnnnn
Awesome
It was a thorough and helpful for the most part but I just can’t understand well because of his accent. Having the captions helped but I had to change video after 1:53. :( Thank you for uploading.
Is Phai or shai?
Psi
Was it always this easy 😶🌫️
arbitaaal
😅
🤣
Voice is good
Here's a question to check whether you are clear with the concept or not...
What are homo and lumo in O2?
অরবিটল
It's excited not exited orbital and ur slang 🤣🤣
Sorry, no homo