Stereoisomers, enantiomers, diastereomers, constitutional isomers and meso compounds | Khan Academy
HTML-код
- Опубликовано: 3 янв 2025
- Courses on Khan Academy are always 100% free. Start practicing-and saving your progress-now: www.khanacadem...
Stereoisomers, Enantiomers, Diastereomers, Constitutional Isomers and Meso Compounds. Created by Sal Khan.
Watch the next lesson: www.khanacadem...
Missed the previous lesson? www.khanacadem...
Organic Chemistry on Khan Academy: Carbon can form covalent bonds with itself and other elements to create a mind-boggling array of structures. In organic chemistry, we will learn about the reactions chemists use to synthesize crazy carbon based structures, as well as the analytical methods to characterize them. We will also think about how those reactions are occurring on a molecular level with reaction mechanisms. Simply put, organic chemistry is like building with molecular Legos. Let's make some beautiful organic molecules!
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Subscribe to Khan Academy’s Organic Chemistry channel: / channel
Subscribe to Khan Academy: www.youtube.co...








I got sick and missed class just as we were starting a unit. Can't thank this channel enough for all it does.
+NeonR3D your welcome mate, cheers!!
In all honesty, this explains it way better than the freaking uni textbook. Or the prep one for organic chem. You guys at Khan Academy can start your own school and I bet there won't be bored students, only curious ones.
this confsed me and i thought i should share, at 11:45 when he says "flip" he really means rotate 180°.
Comment just saved me. :)
I was so confused that he really meant flip like a mirror image of it, i thought why not just flip chiral molecules like that. Thanks tho!
YES THANK YOU
no flipping would be round the axis between the two chiral Atoms, thats just rotating round the molecule center.
i was also confused, thanks!
Flipping means 180 generally, lol!!! 👊👊👊👊👊
Was confused about this for my midterm later this week.....NOW I UNDERSTAND IT. YOU SIR, ARE A GENIUS.
Glad he helped u in your midterm 9 yrs ago :p
10 years passed but this still helps for midterm😂
A couple subtle hints that professors love to TEST on when assigning relationships.
1: if you get stuck, assign R and S names to chiral carbons. If one is SS and the other is RR, its an enatiomer. If one is SS and the other is SR, its a diastereomer
2: Cis/Trans relations apply in these situations. The key to this is to spot a double bond. More then likely the rest of the molecule wont give any information as to what its relationship is, and it will be a diastereomer.
Pro tips mate!
@@breathbadminton lol 9 years ago.. FYI 9 years from now you will have no idea what any of this means. Glad I could help!
@@fleshcookie hahaha I get it yeah
How are you doing in life btw? Glad to see you still have the same gmail acc :p
I hadnt expected a reply.
@@breathbadminton Haha well since I abandoned the pre-med life 9 years ago after college I've lived in 3 different states and worked in 4 different industries from entertainment to management. Currently working in Financial services for the last 4 years. I think about my college time often, and if I had stayed the course to further education like med school. Basically all I can tell anyone debating another 4 years of schooling is you will be amazed at how fast it goes and if you're on the fence just do it. Whatever you choose you will find yourself looking back at this time for years to come so try to enjoy it the best you can. There is a simplicity to the student life that alot of people will try to recapture after a few years in the world. Anyways, best of luck to you.
@@fleshcookie I felt positive after reading about you. Not many people would take their time out to write this. Thanks sir, stay safe and prosperous. Good luck to you too :)
Thanks a lot! I've finished OChem 1 and 2, and never had a teacher that could explain stereochemistry well enough for me to fully understand it. I could usually get the problems right without knowing what I was doing, but that sucks. I get it now!
How are you now mate? :p
dude, you are my fave bengali. you just make my life sooo much easier! pleeeease keep up the awsome job. if you stop, thousands of kids will get kicked out of universities and high schools around the world :'(
So this video released 11 years ago and has been helping since
i spend 3 hours in lectures trying to understand this, i come here and in 13:36 min i understand it all. thanks Sal
This is the hardest chapter I came across in this class :(
yes I have to watch so many of these and do practice
In India we learn this in 10th grade
@@manikantan4809 no, 11th grade
@@manikantan4809 only constitutional isomers in 10th
@@manikantan4809 we literally dont but keep coping
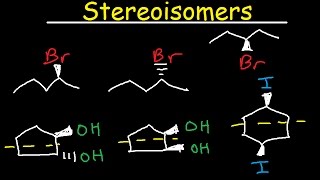
Regarding the last example: the molecule on the left needs TWO rotations to resemble the one on the right. If you rotate it 180 degrees side-to-side, it will have its Bromines on the same side as the molecule on the right, but the Bromines will have dashed lines instead of wedges. But if you rotate the molecule on the left AGAIN, now 180 degrees up-to-down, it will be exactly the same as the molecule on the right. I'm not sure why the instructor didn't mention this.
Doesn't matter - if it is superimposable, it's the same molecule. There are always many sequences of motions that can be applied that end up with the same result. For example, you could flip it three times instead of once around an axis with the same effect.
Sal eres un chingon. I went through the whole organic chemistry series and never really new what a diastereomer or meso cpd was until now.
Oh my man, I missed your voice.
I used this video to study in college, now I am preparing to my prometric exam to leave my home country using this video again😂😂😂
Thanks alot Khan academy you have been a good school all the way
Hahaha omg I'm dying, "Sal wrote superimposible instead of superimposable [12:25]" He's right though, Orgo is super impossible lol love this video though, definitely helped clear things up.
Lina Gomez nice catch
I don't say it now.. it was English captions, right..?
This video is incredibly helpful! I wish you had taught this section of my Orgo course!
Absolutely Awesome! Would it be possible if you could also teach us Biochemistry? I am studying medicine and the failure rate is 85 %,I am already afraid of the test,but with your videos I am sure that I could absolutely pass! Thank you very very much for your effort!
To Abdullah et. al., the molecule is non-superimpose-able because the fluorine on the left side is UP, but when you flip it over and try to superimpose it, it ends up pointing DOWN. The fluorine on the right is already pointing UP, so they will not sync up.
Thanks for correcting a chemistry professors writing mistakes, you must be one of the smartest people on youtube. Hey maybe you'll consider making your own video so you can crack an egg of knowledge on us and enlighten us with your wisdom of words that end in "able." Who knows maybe you'll get a 181,000 views with your awesome vocabular.
@iniloy1993 for your first question, when u put the mirror in the back, the atoms in the back of the actual molecule will appear in the front of the mirror image. and the atoms in the front of the actual molecule will appear father away and towards the back of the mirror image. so that affects whether there is dashes or wedges. If the mirror is to the side.. the atoms in the front will still be in the front in the mirror image and same with the back ones.. i hope that helped.
hey man THNQ> u made my ideas and doubts . clear and all.
Hope u lead a happy teacher life.
Excellent explanations, I understood this enantiomer thing better than reading it in the book. Thank you for your help.
This channel is why I might not have breakdowns before chem exam 😊
This is so confusing. Couldn't the third one be flipped too? I feel like deciding if its a mirror image or same molecule is using a random technique.
It's not confusing. Watch it carefully, you'll prolly understand. Seems cheesy but it's alright.
I agree, from min 4:00,the third one is the same. F and Cl are on oposite sides of the plane and the rest of this molecule is symmetric.
if people started watching this guy instead of going to school everybody would be disburbingly intelligent
College orgo, just started stereochem, so helpful, thanks Sal!
Wonderful...sounds like ma' lecturer watched this video before he teached us Stereo chemistry in previous stage
BEST TUTORING CHANNEL EVER :D
Watched this on 2.0 speed, I have no regrets. Thanks though!
+sebatinilenguini LOL me too!
exam after one hour 😁😁😁
because to have a conformational isomer you need to make a rotation around the sigma bond, naming the different "positions" that the groups have. In this case you're not rotating around the sigma bond, but you're rotating all the molecule in the space or, if you prefer, you're "watching" it from behind.
i may have to do this over - it was a lot of information
Thanks a lot Sal ! You make the most confusing parts of organic chemistry really easy and simple to understand to understand :)
:)
@Iffa Farahin is correct, if a molecule in drawn out of the page then its mirror image will also be out of the page
saved me learning 4 weeks worth of lectures!! xD ta! ty! LOVE IT!
very useful explanation ....helped me a lot .. you deserve a thumps up
You are right in the fact he drew one of the F's in the wrong spot and made them super-imposable, but they still become chiral because non-imposable mirror images do exist now... the mirror image would be from the top or bottom view not the side view though
The molecular formula C4H8O may refer to one of these (not all):
Butanone
Butyraldehyde
Crotyl alcohol
Cyclobutanol
Isobutyraldehyde
2-Methoxypropene
Tetrahydrofuran
i love you, sal!!!!!!
Thank you very much for the amazing video! I had trouble with enantiomers and now I get it completely!
These videos really help.
Thank you so much Sal!
my professor at uconn owes you her whole life savings! thanks!!
ur the best mayn!!
you are a very good teacher
I have one in a week...currently shitting bricks and seeking comfort from khan academy videos.
thank you, I needed this review for my organic chem exam; I was sick the day this was taught :)
thanks for making chemistry ez man respect!
its all about symmetry. when assessing relationships between molecules symmetry should by one of the first things you look for. If there is any possible way you can create a plane of REFLECTIONAL symmetry your done and you know its meso and thus achiral. Also a helpful hint is to look for chiral carbons. If there aren't any it's achiral. My checklist when i do these: 1: it a constitutional 2: are there any C* 3: is there a plane of symmetry. If all those are no its more then likely chiral.
Thank you sir . All my doubts are now cleared
best channel ever
Khan, you're in everything! Keep it up!!!
You know what, I might try that out. Thanks for the advice.
awesome!thank you so much!I just have no clues what my professor is teaching!thank you!~
Thank you so much for these videos! they've been very helpful.
i never thought i would ever understand them.
The chiral center is bonded to 4 different groups. Two groups can start with a carbon and still be different as a whole like a methyl group and an ethyl group.
Who the hell "disliked" this video (or any other video by Sal)?! I really really want to know why?!
CORRECTION!!!!!!
At 11:45, he meant rotate 180 degrees, not flip.
If you flip, the Br will be going into the page. Comments saved me a headache lol.
it helped me alot thnku
Correction 5:07
Chlorine on the right molecule is in the Front and on the left molecule is in the Back
The example at 12:32. Why is it the same compound? If you flip the compound, the angle of the Br and H are NOT the same. In the left you have Br coming towards you and H going back. But when you flip the left one, the Br would go back and H would come in front.
@abclol3kid this is beyond the A-level curriculum
your teacher mainly said that position, branched, and functional are structural isomerism
and the other is stereoismerism
for the purpose of the a-level thats enough, what sal is saying is some more details
I did alevels btw
Your videos are AMAZING! Not only are you saving my keister in organic chem, but you're helping me in molecular bio too! I wish I could send you an edible arrangement!!!
I believe the new semester have come, have you watch all the videos ?
This video is wonderful, thank you.
Great videos for reviewing for my ochem final. Thank you!! :)
Thank you for your video. Extremely helpful.
For the last molecule, the meso compound, when flipping the molecule on the right wouldn't the Bromine on Carbons 1 and 2 be in the back while H would be in the front, meaning that they are not superimposable?
Nice lecture!!!!
LOVE this guy
Hey brother! really good video, I just wanted to let you know that the last two molecules are actually "superposable". To be superposable is different than to be superimposable, any two objects can be superimposed simply by putting one object on top of the other, regardless if they are not the same. To superpose two objects (as in the property of superposition) means, on the other hand, that all parts of each object must coincide. The condition of superposability must be met for two things to be identical. very irrelevant, but just for your own personal knowledge, I know you are busy teaching literally everything on the planet so no biggie. Thanks again for educating America!
Yeah, but no one says it like that and no one teaches it that way either. So, in that regard, Sal is still very much correct.
in the first instance of stereoisomerism he addresses, he calls the 2-chloro-4-fluoropentane enantiomers (07.32) , but they're not are they- if you just rotate the second molecule 180 degrees around the y axis then they become superimposable
you are awesome, if you become an instructor in the future, i'll take your class for sure
Very very beautiful thanks for explanation
Thank you Sal
thank you so much for all the helpful explanations and drawings!
Good explanation. Thankyou sir...................
Thanx so much 😊 4 this video it's help me a lot👍
Good luck!
I disagree on the last example. when you flip the molecule, the Br becomes dashes and it's not the same!
+Cassandra Hanson that was the "aha!" moment i've been waiting to read. i've been so confused about this for a week and i now think i kinda get it. thanks!
+Cassandra Hanson do you want to explain to me what the difference is? the bromine would switch from an axial position pointing up to an axial position pointing down. Rotating it 180 is the same shit.
do not flip, rotate the 2nd one anti-clockwise
This is correct. If you flip them you're looking at the molecule from behind and it looks like enantiomers because the bromines are in the back now. But you can ROTATE the molecule (not flip) 180 degrees to get that exact molecule as well, and thus they are identical molecules but ONLY because they are both bromines. If one was chlorine then it'd be different.
@@vitopettito1689 why would it be different with chlorine? Wouln't you be able to flip the molecule horizontally and twist the carbon with that has the chlorine to get the same molecule?
do you teach somewhere? because i need you as my teacher
THANKS A LOT
very very well explained.
very helpful thanks!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!!
this is a perfect review!!!
Very inspiring figure!
very very helpful and very well explained, thank you!!!
You are the shit dude...I finally understand this stuff ...never did really understand it in general chemistry....I have a test tomorrow and I think you just sealed me a decent grade....I was afraid, very afraid of the" mirror mirror on the wall, what chemistry student is going to take a fall"....HA..Thanks man...
what a great explanation
KHAN: WHY ARE YOU SUCH A ******* AWESOME DUDE?
I second the Biochem comment!
Thank you!!
@tdn1991 You have to think in three dimensions. 2-dimensionally, the drawing doesn't look like it makes sense, but you have to realize that the H and Fl are either going back or sticking out. In the original molecule, the fluorine is coming out at you towards the screen. When he draws the mirrored fluorine, it is going away from you. You can verify this type of behavior in a bathroom mirror. If you move an object toward yourself, its reflection will move away from you and vice versa.
Yes. Mesoforms are the same molecule. It is probably too late for your test, but maybe this will help in the future? :)
9:49 is it safe to just flip the atoms to their opposite direction to see if it an enantiomer?
Is there some quick shortcuts or signs into figuring out if a pair of molecules are enantiomers or the same or etc.? Cause during a test it'll definitely be time consuming to try to visualize a flip and rotation and stuff so just wondering
It wasn't a mirror image, it was just drawn from a different angle
They may look the same, but when you try to superimpose one over the other you will notice that they are in fact different.
great work you make the information so easy ....keep up the good work :)
im kinda confused on couple things..
1) why do you switch the wedges and dashes when the mirror is behind but not switch it if the mirror is to the side??
2) for the last one, if you flip it, then shouldnt the bromines go to dashes rather than wedges?
can we do it? yes we khan!
Got my final tomorrow. Wish me luck, y'all
Good luck