What are Intermolecular Forces?
HTML-код
- Опубликовано: 3 июл 2024
- Chemistry Lesson 5.1
Intramolecular Forces
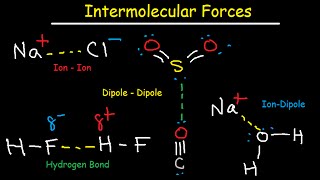
Intermolecular Forces
Ion-ion forces
Coulomb's Law
Dipole-dipole forces
Hydrogen bonding
Instantaneous dipole
Induced dipole
London Dispersion forces
Practice Problems








This video is gonna be the reason the ornaments arent gonna be the only thing hanging this christmas
/j wonderful video xoxo
Wow thank who else is going for exam or Test good luck 💯🤞
You're helping me a lot, dude. Thank you. Keep up the good work!
excellent video illustrations and explanations, really helps me understand IDFs .. THANK YOU
Really dished out the damage that needed to be done! fantastic detail in explaining the major and minor differences between each intermolecular force.
Thank you for helping students with this! Please keep making more videos.
Thank you, I really understood it
thank you so much for this video! I actually now understand what’s going on!! Could you also explain ion-dipole IMFs??
So much good information and very, very clear too 👌👏👏👏
Nice explanation
Thank you sir, Keep up the good work hoping for more videos to upload
Absolutely excellent video thanks a mil!
you're welcome!
@@ketzbook It would be nice for you to cover a complete CHEM course from start to finish. GEN CHEM 1,2 BioCHEM, ORGO 1,2.
Taking notes...
Thanks sir
Hi professor!!!! I think you are amazing and I was literally crying before I found your channel. You helped me understand Lewis structure and gen chem things that I need for my Mcat.
1 REQUEST! Could you please make videos like how you did in the past were it is just the white board and hand on the screen. While you are great to look at, I find that it was much easier to follow your old style of videos for chemistry. Sincerely, a sad student.
Hi, thanks for your message. I have been continuing to make whiteboard animations. Just last month I published one. However, they take about ten times longer to create than videos like this, so I will also be creating more videos that do not require so much time for the animation.
@@ketzbook no problem!! I appreciate the time that you put into these vids. I’m sure you would have a lot of interest in a organic chem series (not sure if you have one) as well as Biochem. Thank you so much again!
I also wanted to say, I love that you explain things in great detail rather than assume that we know everything. It’s what sets you apart from khan academy, professor Dave and many more. Keep making content, you have a wonderful talent and a great mind.
@@ketzbook I think if you would have the time- Mcat focused videos are always a great thing to produce. There’s a huge need & a wide audience. As far as I know, nobody has gone chapter by chapter explaining the Mcat.
@@marye3957 Hi, thanks, I am planning on making some organic chemistry videos eventually, but not biochemistry. What topics for mcats do you think I should cover? I don't know much about that test; maybe I'll look into it.
@@ketzbook functional groups for organic chem would be a great vid.
thank you so much
Thank you very much. It means a lot to me. Why is that? Because it helped me in accomplishing a lot in my exams
glad to hear that!
bro keep it up
My man sounds like his special is lobster bisque.
thank you
Welcome!
😍
Thank you po!!! ❤️❤️
You're welcome 😊
Fabulous video! Question: I thought ionic bonds were stronger than covalent bonds because the ions actually steal or give away its valence electrons? Your video says covalent are stronger than ionic.
I'm actually planning on doing a deep dive into that question later this year. It depends on the the bond, and they are fairly similar in strength. If you do a calculation using Coulomb's law, ionic bonds would be stronger, but that is not an accurate measurement of their strength. The best measurement would be the energy to pull two atoms apart. With that definition, the two are comparable in strength, so it depends on the bond.
I thought Ionic bonds are stronger than covalent bonds because of the electron transfer in ionic bonds rather than shared electrons in covalent bonds???
Me too
you look like steve carell 😃
W
If hero has a face, he looks like this
how is somebody knowing ion ion examples