Chimeric Antigen Receptors: From proof of concept to widely applicable clinical research solutions
HTML-код
- Опубликовано: 21 авг 2024
- Speaker: Dina Schneider, B.Sc.Pharm, PhD, Translational R&D, Cell Biology, Lentigen Technology, Inc., a Miltenyi Biotec Company
Biography: Dr. Dina Schneider holds PhD in Pharmacology and Toxicology from Michigan State University. Her doctoral thesis focused on pathways of B cell activation and differentiation. Her post-doctoral work at the University of Michigan, Ann Arbor, focused on cellular immune responses to viral infections in airway inflammatory diseases. In 2011, Dr. Schneider transitioned to industry, where she contributed to numerous projects in synthetic immunology, immunotherapy, and chimeric antigen receptor (CAR) T cell-based therapy. Dr. Schneider leads the Cell Biology group at Lentigen Technology, Inc., a Miltenyi Biotec Company, in Gaithersburg, MD. Her group is focused on pre-clinical development of novel CAR-based therapies, and on the implementation of CliniMACS Prodigy® platform for preparation of cell-based therapeutics.
WEBINAR: Chimeric Antigen Receptors: From proof of concept to widely applicable clinical research solutions
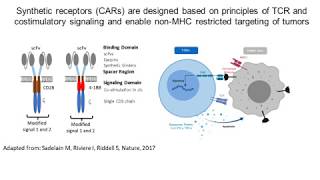
Webinar Abstract: Chimeric Antigen Receptor (CAR)-transduced T lymphocytes have demonstrated impressive clinical activity against B cell malignancies in phase I trials. In most instances, lentivirus-based or a retrovirus-based gene vectors were used to introduce the therapeutic gene expression cassette into the genome of T lymphocytes. The gene expression cassette is composed of an internal promoter and the CAR, created by splicing together of extracellular antigen-binding motifs, linker and transmembrane domains, and intracellular lymphocyte signaling domains. This synthetic engineered receptor can be modified in a number of ways in order to further improve CAR T efficacy and safety. We have found that the combination of two extracellular binding domains is possible, thus expanding the antigens targeted by a single CAR construct, and potentially providing a more effective barrier to the generation of antigen-escape variants. Making CAR-based immunotherapy broadly available to those in need, requires the ability to isolate, activate, transduce, and expand T cell or NK cell populations in a safe clinically-relevant platform. The CliniMACS® Prodigy is a closed cell culture system that combines recent advances in lymphocyte isolation (using antibody-conjugated matrices), activation (using an anti-CD3/CD28 nanomatrix), and automated culture using optimized media and cytokines, into a single platform suitable for the introduction of therapeutic gene vectors, lymphocyte transduction, culture and expansion, and final formulation. Using clinical-grade lentivirus-based gene vectors we have demonstrated the ability to produce CAR-expressing T cells. The CliniMACS® Prodigy platform provides a standardized solution for broad application of CAR-based therapies in clinical studies.
Sponsored By: Miltenyi Biotec
Earn PACE Credits:
1. Make sure you’re a registered member of LabRoots: www.labroots.c...
2. Watch the webinar on RUclips or on the LabRoots Website: www.labroots.c...
3. Click Here to get your PACE credits
Expiration Date: November 07, 2019 10:00 AM
PACE Link: www.labroots.co...
LabRoots on Social:
Facebook: / labrootsinc
Twitter: / labroots
LinkedIn: / labroots
Instagram: / labrootsinc
Pinterest: / labroots
SnapChat: labroots_inc








easily the most comprehensive explaination of this. thank you so much!