How a NanoDrop works
HTML-код
- Опубликовано: 24 авг 2024
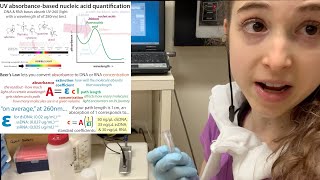
- We often use spectroscopy (such as with a NanoDrop spectrophotometer, which is really quick and only requires tiny volumes) to measure concentrations of molecules like nucleic acids (DNA or RNA) or proteins - by shining light through solutions containing them and seeing how much of the light gets stolen along the way, we can calculate how stuffed full of those molecules the solution was. This is possible because different molecules have different tendencies to absorb different wavelengths of light to different extents (more in a sec). If you shine a molecule’s “favorite” wavelength at it, the more molecules there are, the more that will get stolen. You could just measure the absorbance of this favorite wavelength and, if you have a pure sample and you know how much your molecule likes that wavelength (the extinction coefficient) you can calculate the concentration (using a law called Beer’s law) just with using that one wavelength of light.
But is your sample really pure? If other molecules like that wavelength too they can be artificially inflating your concentration - not to mention other problems with having contaminating molecules around that can interfere with things.
So (if possible) you shouldn’t just look at a single wavelength absorption value - instead, you can learn a lot more if you measure over the whole ultraviolet (UV) and visible light portions of the electromagnetic radiation (EMR) spectrum. Each wavelength that gets absorbed will show up as a peak an absorbance spectrograph, which shows how much light of a range of wavelengths was prevented from making it all the way through the sample to the detector on the other side.
for a longer version: bit.ly/dnauvbeer
The height of the peak you usually focus on for a particular molecule is where it absorbs best. This corresponds to how much stuff is there (concentration) (taking into account that molecule’s willingness to absorb that wavelength (its extinction coefficient). BUT a lot of information about purity can be gained by looking at where they shouldn’t be absorbing much light - and ratios between peaks can tell you about how pure that stuff is
You can see this if you look at a UV-Vis absorbance spectrum, which you can can get if you use a spectrophotometer like a NanoDrop. To get a full absorbance spectrum, you shine light of all wavelengths (well, at least all visible & some ultraviolet (UV), which is where biological molecules tend to absorb) & measure what goes through (is transmitted). Whatever doesn’t go through is assumed to be absorbed (or abducted by aliens…). So absorbance = 1-transmission.
So how does it work? We take whatever solution we want to measure and put it in a plastic or glass holder called a cuvette which has a window for light to shine through & stick it in a spectrophotometer which actually shines the light through one side & measures what comes out the other. Different cuvettes can have different path distances the light has to travel, and the Beer Lambert law takes this into account - the longer the path, the more molecules light’s likely to hit (regardless of the concentration) - and the more molecules it hits, the more chances there are to be absorbed. Our calculation of concentration is based on how much light gets absorbed so we need to account for this distance.
It’s also important to have a blank - this is just the liquid you have your samples in and all the “constant” components (e.g. salts, etc. that are in each reaction) - its made up of molecules too so it’ll have a characteristic absorbance that will always be there, whether or not the reaction we’re looking for actually occurs. And some of its absorbance spectrum might overlap with our products’. So we want to subtract it out so we don’t confuse it for our signal.
Cuvettes are great for things where you have “large” samples. But if you don’t have much sample though, you’ll want something smaller-scale.
The NanoDrop spectrophotometer has a little pedestal you put a drop of liquid on (a really tiny drop, like 1-2μL “μL” stands for microliter and it’s a millionth-of a liter, or a thousandth of a milliliter. Then you lower the arm → it contacts the liquid then pulls up a little bit and, when it does, it pulls on the liquid. It does this thanks to surface tension. Surface tension occurs because the molecules of the liquid like each other more than they like the air - so they try to stay together & maximize the liquid-liquid interactions while minimizing their combined air exposure. more here: bit.ly/surfacet...
When you put the drop on, surface tension causes it to remain drop-like. But when you lower the arm & squish it down, some of the water molecules stick to the top surface. And when the arm pulls back up, these molecules get lifted - and the other water molecules don’t want to leave their friends behind → as a result a column of liquid forms
finished in comments







This column is just like the column of liquid in the cuvette except it’s much smaller and it’s held up by surface tension instead of being barricaded by glass/plastic. And it’s “sideways” - the light travels through from above and is recorded below
Another difference about this column is that the path length’s adjustable (the column can be pulled up higher or squished) - so it can adjust for different concentrations (e.g. if your sample’s too concentrated it’ll see this squish down to shorten the path length so the light doesn’t meet as many, or if it’s too dilute it can pull up to make sure lots of molecules get hit by light). The NanoDrop software then has to correct for this when it uses Beer’s Law to get to concentrations
A couple things we commonly use it to measure are nucleic acids and proteins. It’s easier to get columns to form for nucleic acids than proteins because the proteins often weaken the surface tension - not all parts of proteins like water, so it’s harder to get continuous water networks to stay as you pull up the column → column breaks. But there’s strength in numbers, so, when loading protein, I usually load 2uL, but I load 1 or 1.2 for nucleic acids.
Also, beware that if the concentration is too low, the results will be unreliable, especially the ratios. If you need really accurate concentration info, a Qubit is a better option - it uses fluorescent molecules that bind specifically to DNA, RNA, or protein, depending one which kit you use.
much more on how to interpret the results: bit.ly/dnauvbeer ; RUclips: ruclips.net/video/cUxh2DCqYBo/видео.html
more on UV absorption of proteins: bit.ly/tryptophanfan ; RUclips: ruclips.net/video/lcnmE4TGt7I/видео.html
suggested reading: What Causes Molecules to Absorb UV and Visible Light. (2020, August 21). Truro School in Cornwall. chem.libretexts.org/@go/page/3746
The NanoDrop manuals also has good info if you want more. Hope this all helps!
more about all sorts of things: #365DaysOfScience All (with topics listed) 👉 bit.ly/2OllAB0 or search blog: thebumblingbiochemist.com
Thank you, I couldn’t find a lot of Info on this subject on RUclips until I found your content ☺️
Glad you found it helpful!
Easy and detailed!!
It's interesting how persepctives can be so different. The way I look at it, the DNA is contaminating my protein sample!
Sometimes I feel that way too!
Well explained, and very helpful, thank you very much!
Glad it was helpful!
Thank you for the information!
Nice detailed explaination! Great Job!
explanation!
thanks!
Quick question. For DNA QUANTIFICATION, the more fragments of DNA there is in the sample the higher the nanodrop value it will be correct? Thus if I am digesting DNA from an initial known concentration of DNA, I expect the value of the nanodrop to increase as there will be more fragments of DNA for the spectrophotometer to read and measure. Nice video btw keep up the good work
No. It's concentration in terms of mass/volume, which won't change when you digest it. And thanks!
Awesome thank you!
Hey, this explanation was great ! But tt'd be also awesome if you could make tutorials on these machines/techniques like "How to use Nanodrop to dilute samples" and explain the procedure like we are five years old. Just an idea tho.
thanks. maybe if I find time...
I only survive in the lab thanks to you!
So happy I could help! Best wishes!
Thanks Thanks Thanks ❤❤❤❤❤
Big fan madam ❤
Thank you!