Functional groups | Alkanes, cycloalkanes, and functional groups | Organic chemistry | Khan Academy
HTML-код
- Опубликовано: 30 сен 2024
- An overview of some common functional groups.
Watch the next lesson: www.khanacadem...
Missed the previous lesson? www.khanacadem...
Organic Chemistry on Khan Academy: Carbon can form covalent bonds with itself and other elements to create a mind-boggling array of structures. In organic chemistry, we will learn about the reactions chemists use to synthesize crazy carbon based structures, as well as the analytical methods to characterize them. We will also think about how those reactions are occurring on a molecular level with reaction mechanisms. Simply put, organic chemistry is like building with molecular Legos. Let's make some beautiful organic molecules!
About Khan Academy: Khan Academy offers practice exercises, instructional videos, and a personalized learning dashboard that empower learners to study at their own pace in and outside of the classroom. We tackle math, science, computer programming, history, art history, economics, and more. Our math missions guide learners from kindergarten to calculus using state-of-the-art, adaptive technology that identifies strengths and learning gaps. We've also partnered with institutions like NASA, The Museum of Modern Art, The California Academy of Sciences, and MIT to offer specialized content.
For free. For everyone. Forever. #YouCanLearnAnything
Subscribe to Khan Academy’s Organic Chemistry channel: / channel
Subscribe to Khan Academy: www.youtube.co...


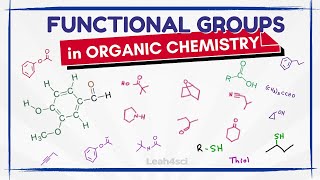






Why is the thiol group not called an EthylThiol????? :(
+Dej Green because they aren't following the iupac standard
Dej Green why crying for it?
I don't get the ether joke XD
you mean.... I didn't get the joke ether? haha
i dont get the joke 😂
Because the ether has an R group on either side of the oxygen..... Either sounds like ether
Hindi me khaa hai
Explain in hindi 🙋
Which functional group does propanon have?
I didn't notice the bad joke till you pointed it out lol.
isn't ethyl suppose to be triple bonded???
What does he use to draw/type in?
so do we just say diethyl when theres two ethyl groups?
That should be butane
What means the dots under or above the element? The pair of free electrons?
i think the dots stand for the electrons the atom has on its valence shell that are not bonded in a covalent bond with another atom. for example in the video alkyl halide Cl shares two electrons with a carbon atom and has six electrons that are not bonded with any other atom (and we see those as the six dots in the video).
It helped a lot
thanks
Thanks!