Organic Chemistry Elimination Reactions - E1, E2, E1CB
HTML-код
- Опубликовано: 6 сен 2024
- This organic chemistry video tutorial focuses on elimination reactions of alkyl halides and alcohols to form alkenes. It covers E1, E2, and the E1cb reaction.
Stereochemistry R/S Configuration: • Stereochemistry - R S ...
Optical Activity & Specific Rotation:
• Optical Activity - Spe...
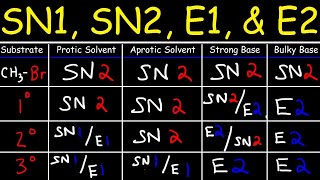
SN1, SN2, E1, E2 Reaction Mechanisms:
• SN2 SN1 E1 E2 Reaction...
SN2 Reaction Mechanisms:
• SN2 Reaction Mechanisms
SN2 - Test Question:
• SN2 Reaction Mechanism...
_______________________________
SN1 Reaction Mechanisms:
• SN1 Reaction Mechanism
Carbocation Stability - Hyperconjugation:
• Carbocation Stability ...
Carbanion Stability:
• Carbanion Stability
Protic Vs Aprotic Solvents:
• Polar Protic Solvents ...
E1 Ring Expansion:
• E1 Reaction Mechanism ...
E2 - Test Question:
• Zaitsev vs Hoffman's P...
________________________________
E2 Stereochemistry - Newman Projections:
• E2 Stereochemistry Wit...
SN1, SN2, E1, E2 - Practice Test:
• SN1 SN2 E1 E2 Reaction...
Organic Chemistry PDF Worksheets:
www.video-tuto...
Organic Chemistry 1 Exam 2 Playlist:
bit.ly/3PKEApB
Full-Length Videos and Worksheets:
/ collections







Organic Chemistry PDF Worksheets: www.video-tutor.net/orgo-chem.html
Full-Length Exams and Worksheets: www.patreon.com/MathScienceTutor/collections
ppp
You've taught me through out premed and next semester I'll be done with premed and its all thanks to you and AK lectures, another tutor on youtube. Thank you so much.
this guy is a legend but one thing even when my volume is at 100 he's still low lol
Yeah😂😂
True
Whenever I see your background format, I immediately click on your video
I've been watching for years now and I usually get an A
Thank you for taking your time
Thank you a LOT for all these great videos!!! Not everyone is made to teach but you are a natural talent! Keep the good work going :)
excellent video... was uploaded 5 hours before my test yesterday. saved me. plz make one on organic halides too.
Chances are, I made one already. When you say alkyl halides, do you mean SN1 and SN2 reactions of alkyl halides or something else?
+The Organic Chemistry Tutor
organic halides include following topics:
-their preparation
-physical properties
-their reduction
-aryl halides which includes sandmeyer reaction,wurtz fittig reaction, fittig reaction, ullmann reaction
-polyhalogen derivatives including their preparation and many important reactions like carbylamine reaction, reimer tiemann reaction etc.
chloroform, iodoform
-allyl halides
-GRIGNARS REAGENT
Unfortunately, I don't think I'll be making a video on those topics any time soon :)
Imo this series is better than crash course's. I just understand this one better! Thank you!
as a jee aspirant i literally recommend u to watch this man to clear any doubt or even if u are studying for the first time
Agreed! As a non Hindi speaker, this guy and prof Dave are my go to for anything remotely related to chem
14:23 the more accessible hydrogen **
Thanks from Egypt
good ears. i almost wrote the wrong info lol
Thanks!!!! Your videos are the reason I'm passing Organic Chemistry
You are LEGENDARY! This video helped me so much, I never understood why multiple products are formed until now. You are amazing!!!!
you are the best, you made organic chemistry friendly. much love from Ghana.
thanks a lot..it finally cleared my concept of elimination reactions
immensely helpful, thank you so much.
I am in class with my earphones and my lecturer is trying so hard to explain 🙃 LOL am in my own class ooooh😜🤛🤜I am about to explain to the class thanks sir salute you🇰🇪🇰🇪🇰🇪
Perfect and the most understandable as always 🙌
Thank you so much...your videos are life-savers!
At 30:50, During the E1 reaction mechanism , would a hydride shift occur from the green H, shifting the carbocation to where the hydrogen previously was, leading to a more stable carbocation? Your videos are truly so helpful thank you so much!!
yeah that shift will happen but because of symmetry you will get the same product while removing H so ultimately 2 product
Superb explained... Keep uploading video like this
May God bless you man!! You are blessing for my grade
Best online tutor
all your videos are more than helpful , thank you so much
BEST VIDEOS ON WEB !!!!! ^-^ THANKS A LOT BRO great job
Excellent teacher with clear perfect presentation , thanks a lot
how can you distinguish an e reaction from an sn reaction? in the event that you're only given the initial molecule and what it is reacted with and asked to predict the product.
okay so
(1) If given R-OH with Conc. H2SO4 or Conc. H3PO4 then you know it is E1.
(2) If given R-X with H2O+Acetone(for solubility)/ROH (heat) then SN1 can happen along with E1. Generally SN1 one will give major product and elimination will give minor if in question not given about they are talking about elimination product or substitution based product only.
Here H2O acts as a base in elimination and Nucleophile in substitution. so you can take others also in aprotic or protic solvent accordingly.
(3) Pinacol - Pinacolone E1
(4) You may notice from above that E1 happens in an acidic medium.
(5) Elimination reactions are generally slow reactions and require heating during the process. you may have listen this, generally addition>substitution>elimination for rate.
(6) E2 and E1cb occur in basic medium.
There are some examples where elimination products are more than substitution like
Eg1:- 2-Iodo-2-Phenyl-butane with KOH here SN2 is not favourable due to 3 degree halide so E2 give major product.
Eg2:- 5-Bromo-cyclohexa-1,3-diene with KOH here you will get benzene by E2 so it give major product rather than E2 (solvent is a protic)
might be helpful i guess
you may also refer to peter sykes chapter 9 elimination reaction part 9.5 elimination vs substitution.
Thanks for clearing my concepts
This one video cleared up so much for me, thank you
Your videos are a great help to
Me
Thank you so much
Simply the best
No long talks❤️
7:50 - As far as I know, the final product needs to be somewhere between the intermediate and the rractant on the potential energy diagram as the overall process is indeed endothermic. That would also clearly depict that the activation energy of the first step is higher than the second one. Please check it out and let me know if I am wrong
Your videos are amazing. Thanks a lot
Thank you very much, professor 🙏🏽
Thanks alot for the video , and definetly a great admirer of your teaching!!
At 53:09; is a rearrangement (hydride shift or is it really preferred over methyl shift even in THIS case) going to take place to produce a more stable tert- carbocation intermediate;
and there after would we be getting a sulphonic acid group attached;
or is this whole thing a dehydration of alcohol resulting in the formation of a double bond?
I am still a rookie in this so please help me out!!
Always grateful :)
AT 52:00 can we have a hydride shift instead of metho shift, by the way ur videos are too awesome to be complemented.
Very best explanation
jee (india)
aspirants mark your attandance
thanks i really need to get this down because it my 3rd time taking ochem
I can not thank you enough ♥️👏
6:23 what about stability of things like n,n di alkyl teriminal akenes wrt. cis and trans dialkyl substituted alkenes
how can you tell if something is a poor leaving group?
Thank you...thank you .....thank you so much
Thank god for this man
Thank you so much. Your video is awesome.
Why doesn't the oxygen in the water goes for the carbocation instead of a hydrogen?
Thank you!
35:47 addition or elimination?
22:38 could we also say that the product on the left would be formed because the hydrogen removed would be an acidic hydrogen ??
no, check it by product stability. we would say this if the case is of Hoffman in which there are leaving groups like -NR2(+), -SR2(+), -F.
for eg take 2-Bromo-butane here 2* hydrogen is removed to give a major product rather than 1* hydrogen because here major product has more hyperconjugation.
(Here 1* hydrogen is more acidic since carbanion stability is 1* > 2*)
* = degree
if you find mistakes then please let me know
thanks for another hitter
That was great!
saving lives out here
Thanks
Thank you!!!
This banged
thank you sir :)
I LOVE YOU!
Saving my degree frfr
Tertiary carbocations are more stable than secondary no?
Minefortress21 yes they are (responding to a question from 6months ago, wondering whether you've been patiently waiting for a response all this time :)
Yup. Stability depends on the number of alkyl groups (Ch3) attached to the carbocation.
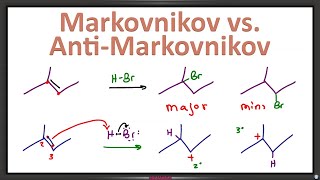
14:40min : is the base bulky it is going for the *more accessible hydrogen :)
or less hindered
والله انت راجل توب التوب
still no benzene rings smh
Thank you
I love ou
Who understand well type❤
2:38
I can't hear it ;-;
👏👏👏👏👏👏👏
Can someone who knows this topic very well send me their discord there is one question that has some controversy around it and I want to know what the correct answer of that should be. It's based on elimniation reactions
"you can't remove the green or the purple hydrogens because it's sin"
🌹🌹🌹
in this video 🥺 🤭
Fuck yeah
pp
Awwwwwww😅🥴
👍👏👏👏👏👏👏👏
Why do you ground letters when you talk, that wouldn't make you sound more manly
pretty mid video fr 🥱🥱🥱
I thought the tertiary carbonation is the most stable?
Ohh sorry I got lost In the vid 😅😅
thanks a lot..it finally cleared my concept of elimination reactions