Explain the principle of Fluorescence and Phosphorescence. | Analytical Chemistry
HTML-код
- Опубликовано: 9 фев 2025
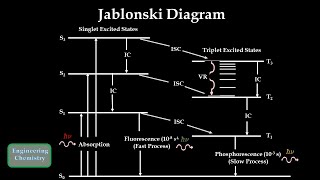
- Many compounds absorb ultraviolet or visible light and undergo an electronic transition from low electronic energy levels to high electronic, energy levels. This Absorption of light requires 10-15 sec.
The instant re-emission of the absorbed energy is called Fluorescence.
While delayed re-emission of the absorbed energy is called Phosphorescence.
For most molecules, the electrons are paired in the ground state i.e. pair of electrons have opposite spins this is called singlet state. As the spin is paired up, they do not show any magnetic moment.
But if the electrons have the same spin the magnetic moment of both the electrons gets combined and they behave like a tiny magnet. This state where the electron pair is having the same spin is called as triplet state.
In a molecule, there are several electronic energy levels and electrons are present at the ground state in the singlet state i.e. with opposite spins. When a molecule absorbs U.V. or Visible light, one electron from ground state undergoes to an excited state. Now the excited state is also having several vibrational levels, so the excited electron loses its energy by intermolecular collision and it comes to the lowest vibrational level of the excited state. Finally, the electron from the lowest vibrational level of excited state returns to the ground state by emitting the radiation or light of lower energy or higher wavelength.
This overall process of absorption, intermolecular collision, and emission take 10-4 to 10-8 sec which is very small thus it looks like instant re-emission of light. But the energy of radiation emitted is always less than the energy of radiation absorbed. Or in other words, the wavelength of emitted radiation will be always higher than the wavelength of radiation absorbed. This is Fluorescence phenomenon.
Analytical Reasoning
• Solve Problems Related...
English Grammar
• Making Sense With Tens...
Interview Skills
• Questions You May Be A...
Managerial Economics
• MCQ #1 of Managerial E...
Royalty Free Stock Footage
• Flowers - Royalty free...
Chemical Thermodynamics - Physical Chemistry
• State and Explain firs...
Ionic Equilibria - Physical Chemistry
• Derive expression for ...
Electrochemistry - Physical Chemistry
• What are different typ...
Solid State - Physical Chemistry
• Explain the following ...
Gaseous State - Physical Chemistry
• Postulates of Kinetic ...
Colloidal States - Physical Chemistry
• What is Colloidal Solu...
Stereochemistry - Organic Chemistry
• Explain Configuration ...
Nanomaterials - Engineering Chemistry
• Compare top down with ...
Water and Its Treatment - Engineering Chemistry
• Explain why hard water...
Electrochemistry - Engineering Chemistry
• Distinguish between me...
Environmental Studies
• MCQ on Environmental S...
Optics - Applied Physics
• What are cartesian sig...
For Details Visit
cepekmedia.co.nf
cepek.hol.es/
edmerls.66Ghz.com/
edmerls.tk/








Wow, very good video, congratulations :)
(I am a colleague from "The Cube", Rizzi ;-) )
Are you from Udine?
Just amazing 👍
Bestest brief or compact explanation...
Well explained in less amount of time!!
very well explained
Amazing visualization !!!
Great video! Wish you explained why the spin becomes swapped in phosphorescence. Also are infrared photons emitted as well?
Nice video to quickly brush up on the concept thank you
Thanks, It's Well explained and easy to understand
The explanation is good, but what's the music for? It's distracting. Would have been much better without any background noise.
Oh
Very good explanation of the trickiest part in short time
Woow!!! That was great
You forgot to reinverse de spin of the electron when it comes back to the ground (singulet) state
Amazing..so clear and nice
I read the Wikipedia article on this first. I did not understand it. The graphics in this video did a great job of explaining the top levle ideas, but now I have more questions. One obvious one is why does the spin flip in some situation and not in others?
Really very much inspired by you sir... I had been watch your videos from my 3rd Yr bpharm.... Thankyou for your easiest explanation... #PharmaCtutorial
If you like we can work together..
@@Edmerls in what way we can work together sir..?
cepekmedia@gmail.com is my email.. write to me and i will explain.
Super explanation..... Loved it.
Amazing 😍
Thank You!
Great video!! Thank you :)
Excellent explained
Glad you think so!
Awsome explaination:)
Very good explanation 👍👍👍👍👍
beautiful vedio
make videos on electrode processes and kinetics
Very well explained by diagram👍
Thanks and welcome
very helpful, thank you so much
Thank you very much! Regards
Thank u sir
What happens when we transfer slightly higher energy that causes both electrons from the singlet state to move to an excited state?
Your video is very cool.Keep it up.
best explanation
Really thanks 🙏🌹
So nice of you
can you give the name of the reference book
It was an excellent video. What was the last sentence in the video ? It was something about the “virulent of the energy absorbed is less that the energy emitted...” I might be wrong
Nice
Good and precise explanation 👍
Thanks
Super
Thanks
Welcome
i LOVE IT ! THANK YOU
CHEERS FROM MEX :)
Thank you
The diagram is INCORRECT and wrongly depicts the excited electron in the triplet state falling back to the ground state without flipping its spin, and thereby violating the Pauli exclusion principle! The whole reason phosphorescence occurs is because you have to wait for the spin to flip while still in the excited state BEFORE it can fall back to the ground state.
What is the difference between emission in fluorescence and AES. I DIDN'T get it
@@nayudugaya3816 fluorescence refers to the excitation and subsequent light emission of molecules, usually in a solid state or liquid system; atomic emission spectroscopy refers to the absorption and emission of light from single atoms in isolation in a gas. In AES the excitation wavelengths are always identical to the emission wavelengths because the atoms are in isolation and there is no other mechanism available for energy loss other than a photon emission. It's therefore a very simple system. In fluorescence, there is much more complexity, with the excited molecule in eg. the solid state there is ample opportunity for energy loss to neighboring atoms and molecules by eg. crystalline lattice vibration (phonon) coupling, and even when molecules are isolated in space such as in the vapor phase, there is the ability for the molecule to undergo internal vibrational mode transitions which reduce the energy state before fluorescence emission. Thus in fluorescence there is always a Stokes shift of the emitted light compared with the excitation light, ie. energy is always being lost somewhere before re-emission takes place.
@@Muonium1 thanks for ur gesture
So can we say like solid and liquid systems analysed by fluorescence and gas by AES.... kindly reply plz
@@nayudugaya3816 to answer your other question I am an optics engineer at a laser-driven nuclear fusion research facility. Loosely and generally speaking you may say that, yes, but very strictly and scientifically speaking, no, we cannot say that for the following simple reason: molecules can exist in the gas state. For instance you may examine the yellow fluorescence spectrum of iodine by exciting the I2 molecules in the vapor phase with a green laser beam (search for "iodine fluorescence" to see videos here) and we can observe the fluorescence of things like C2 molecules in the tails of comets, in the atmospheres of certain 'carbon stars', and using microwave spectroscopy of organic molecules in the interstellar medium. Also, "solid" materials, for instance rocks, may be analyzed by AES by vaporizing them first using a laser beam in a technique called LIBS or laser induced breakdown spectroscopy, where tiny amounts of the material are converted into a plasma or very hot gas in order to essentially be analyzed by what is effectively then AES.
Thank you so much sir. Can you share me an XPS old data, I want to practice it.
I have also started journey of making things easy for others
Thank you for the easy explanation :D
Then what is the role of doublet in fluorescence
Thank you, I am trying to understand phosphoresce just for the sake of understanding how my calcite rock works.
good luck
Very well explain sir please aur analaytical chemistry ke videos daliye MSC sem fourth ke
what does intermolecular collision means?
Hello, how can two electrons be in the same quantum state at the end of the phosphorescence process? Shouldn't one of the electrons change its spin state according to the Pauli principle?
they can't, the diagram is wrong. he wrongly depicted the phosphorescence decay as violating the Pauli exclusion principle.
Woow 😮😮
Yes, I had difficulty at first, but I kept listening and I could understand him pretty well.
danx sir
Very nice except for the part at 2:10 - "Wavelength of emitted radiation will be always higher than the wavelength of the radiation absorbed." Should be "always lower/longer."
which one will show more stroke shift flourescence or phosphorescence
What kind of substance undergoes phosphorescence
Then how does it describe, transition from Singlet1 to Triplet1 state ie S1 to T1 and S2 to T2 ?
The video is well done. However, the accent is very strong and thus it is difficult to understand what was said. Please include subtitle in the video!
Thanks alot
Just owo
thanks a lot
Thanks sir
Show that stark Einstein law is the quantum mechanical equivalence of grothus draper law
explanation 👍
Weird background music, but actually very good instruction lol
It is infinite glow of finites.
👌
Why is fluorescence so much sensitive
Utkrisht
What was the point of the background music?
Dear bro please check practical ninjas channel some corrections r here...Tq...particularly about spin change...
Hlpful
what the hell with the music
It's just a bit too loud.
Thank You