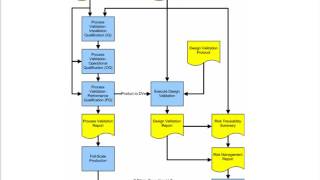
Process Validation Procedure for Medical Device Manufacturers
HTML-код
- Опубликовано: 9 сен 2024
- This Process validation training/webinar for medical device manufacturers will discuss the CDRH interpretation of the GHTF guidance and how manufacturers should develop Process Validation Plans and conduct production Process Validation.
For More Information Contact -
Organization: NetZealous BDA GlobalCompliancePanel
Website: www.globalcompl...
Email: support@globalcompliancepanel.com
Help us caption & translate this video!
amara.org/v/Qnms/