What is Colloidal Solution? | Colloidal State | Physical Chemistry
HTML-код
- Опубликовано: 9 сен 2024
- Download PDF Notes at edmerls.com/wha...
The colloidal state of matter was first introduced by Thomas Graham in 1861. He divided substances into two classes, depending on the ability of diffusion.
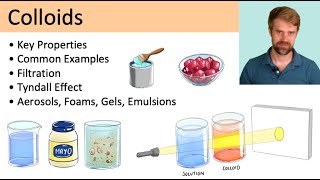
Colloids are the substances which can not diffuse through colloidal membrane or parchment membrane e.g. starch, gelatine etc. if the particles of sugar are dissolved in water, a homogeneous solution (true solution) is obtained. The particles in true solution are too small (10-9 m) to be seen with naked eye or even under microscope.
On the other hand if a little clay or sand is mixed with water, a heterogeneous mixture (suspension) is obtained. The particles in suspension are so large (10-7 m) that they are visible even to the naked eye.
The particles in colloidal state possess the size between that of particle in true solution and particle in suspension.
In other words the size of the colloidal particles lies between 10-9 m and 10-7 m.
The colloidal particles form the dispersed phase, the medium used for dispersion is called dispersion medium.
A dispersed phase & dispersion medium together form a colloidal system.
The dispersed phase and dispersion medium may be solid, liquid or gaseous.
For Details Visit
cepekmedia.co.nf
cepek.hol.es/
edmerls.66Ghz.com/
edmerls.tk/








thanks you just saved my chemistry essay.
Good explanation sir
Kuch samjh me nahi aya bas likhaye hi ja rahe hai read karte ja rehe hai kuch samjh nahi rahe hai
collidal state matlab kuch beee nehi deke ga...ouuur
suspension matlab thoda thoda dekhe ga ouur
true matlab saf saf dekhe ga
Really interesting 😊
Is collidal solutions missible or not plzz ans
Thanks sir 👍
Thnx sir
Very helpful sir
It's really very interesting and 👍🙂
Live chemistry classes. On the spot doubt solving. ruclips.net/video/SjH0D-hZeus/видео.html
Nice bro😀
Nice bro thank you 😁😀😙
☝️👍
Colloids and solution allaj hai to colloids solution kyo kaha
Aise hi bolte hai.. solution ko actually TRUE SOLUTION bolte hain.
Fuck You
Plz don’t make video
Thnx sir
You are welcome Innama!