Equilibrium Constant K & Cell Potential Problems With Ksp - Electrochemistry
HTML-код
- Опубликовано: 9 сен 2024
- This chemistry video tutorial explains how to calculate the equilibrium constant K value given the cell potential using a simple formula. It also explains how to calculate the cell potential of a half reaction given the ksp of another chemical reaction. This electrochemistry video tutorial contains plenty of examples and practice problems on the equilibrium constant K and cell potential. For a spontaneous reaction, the change in giibbs free energy is negative, the cell potential is positive and k is greater than 1. For a nonspontaneous reaction, the change in free energy is positive, the cell potential is negative and k is between 0 and 1. For process at equilibrium, the change in gibbs free energy is zero, the cell potential is zero, and k = 1.
Intro to Galvanic & Voltaic Cells:
• Introduction to Galvan...
How To Draw Galvanic Cells:
• How To Draw Galvanic C...
Standard Reduction Potentials:
• Standard Reduction Pot...
Cell Potential Problems:
• Cell Potential Problem...
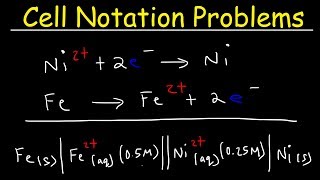
Cell Notation Problems:
• Cell Notation Practice...
___________________________________
Concentration Cells:
• Concentration Cells & ...
Cell Potential & Gibbs Free Energy:
• Cell Potential & Gibbs...
Cell Potential & Equilibrium K:
• Equilibrium Constant K...
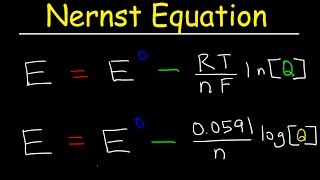
Nernst Equation:
• Nernst Equation Explai...
Electrolysis of Water:
• Electrolysis of Water ...
_____________________________________
Electrolysis of Sodium Chloride:
• Electrolysis of Sodium...
Electrolysis & Electroplating Problems:
• Electrolysis & Electro...
Electrochemistry Practice Problems:
• Electrochemistry Pract...
SAT Chemistry Subject Test Review:
• SAT Chemistry Subject ...
Carbon -14 Dating:
• Carbon 14 Dating Probl...
Beer Lambert's Law:
• Beer Lambert's Law, Ab...
______________________________________
Final Exams and Video Playlists:
www.video-tuto...
Full-Length Videos and Worksheets:
/ collections
Chemistry PDF Worksheets:
www.video-tuto...







Next Video: ruclips.net/video/jousNNceCXs/видео.html
Chemistry PDF Worksheets: www.video-tutor.net/chemistry-basic-introduction.html
Final Exams and Video Playlists: www.video-tutor.net/
Full-Length Math & Science Videos: www.patreon.com/mathsciencetutor/collections
dawgs been saving my life since '15
In this problem 4:18
this method of calculating the E of the third equation by adding the other two only works when n is the same amongst the three of them (n is the number of transferred electrons)
otherwise you should use
n3E3 = n1E1 + n2E2
Great video, in spanish there isn't good videos of electrochemistry. This is a great chanel.
you saved me again man, I was wondering where did that K came from in my module, thanks
Really good explanation 🤞🏾
Great lesson
4:07 how would I even put that into the calculator
Why are you not able to flip E* of cranium? can the volts not be negative?
What is the value of "e"?...I couldn't get what u said concerning it
i just wonder how the organic chemistry looks like hey
What's the value of e
Please I need answer urgently
Nice vid! Btw, what's your educational background?
EVERYTHING lol
What's the value of e please respond
1O^n ....for eg. 2e4= 2x10^4
❤
what is the value of e ?in k=e (nFE°)/RT ?
What's the value of e
It referring to the e^x, the "e" value is just the inverse of ln and on your calculator it would be the e^x which is better stated as e to the x power and x is the (nFE)/RT so whatever the number get for the (nFE)/RT part of the problem take that number and use the e^x button (plug that number into where the e is at) and you will get K
its a euler
e ≈2.71828182846
in 0:32 why did you said that if use -0.73 V wont work?
Yana Gida Because it’s a voltaic cell that means it should be spontaneous and it can’t be spontaneous if has a negative Ecell so -0.73 won’t work .. You have to get a positive Ecell when you add the Eanode and Ecathode for the reaction to be spontaneous.. I hope this helps🙏🏾
@@ugochionuoha4853 Thankss! finally understand it!
wouldnt the Ksp= 1/K?
no