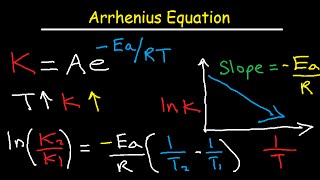
Creating an Arrhenius plot to find Ea
HTML-код
- Опубликовано: 27 июл 2024
- This video shows you how to do an Arrhenius plot from temperature and rate data and how to find Ea.
Ea = activation energy, in J/mol (or kJ/mol if you want to convert it into this)
A = pre-exponential factor (frequency factor). For units, see notes at bottom.
Ea is the minimum amount of energy needed for a reaction to occur.
A is the frequency of collisions between particles, in a reaction. It is the fraction of molecules that would react if there were no energy barrier, ie if activation energy were zero.
A large A value = more frequent collisions, the reaction happens more easily.
A large Ea value (steeper gradient) = a higher energy barrier, the reaction does not happen easily
WATCH NEXT: Forms of the Arrhenius equation you need • Video
Note - units of A can differ depending on the order of reaction. This is quite complex and above and beyond what you need for A Level. Units of A are often given as s-1 for simplicity.
A is temperature-dependent, however if a narrow temperature range is chosen, we can assume that A is constant for two reactions at different temperatures.
Music: I Never Stop To See You Crying
Musician: Belair
URL: icons8.com/music/



![Finesse2Tymes - Pretty Ricky [Official Music Video]](http://i.ytimg.com/vi/xIoP_mhYRT0/mqdefault.jpg)
![Cordae - Saturday Mornings (feat. Lil Wayne) [Official Music Video]](http://i.ytimg.com/vi/z2GYJua09F0/mqdefault.jpg)



Thank you so much
You're welcome!