Make Copper Metal from Aluminum Foil - Our #1 Home Chemistry Experiment
HTML-код
- Опубликовано: 30 июн 2024
- In this chemistry experiment video, we take you step by step how to make elemental copper metal from aluminum foil and copper sulfate, and explain all aspects of the procedure & chemical reaction in detail.
This experiment ranks #1 in our list of home chemistry experiments, because of the ease of its procedure, ease of getting the necessary materials, dramatic results, usefulness for future experiments, and lack of corrosive acids or bases used.
Because copper sulfate is toxic, it must be handled by adults only and stored in a place secured from kids. It is important copper sulfate is not ingested, inhaled or in contact with the skin.
Chemical reaction: 2Al + 3CuSO4 → Al2(SO4)3 + 3Cu
Expected yield of elemental copper is 63.55*(50.0/249.68) = 12.7 grams.
After 2 days of drying, we ended up with 12.65 grams of Cu, for a fantastic yield of 99.6%.
ChemTalk website: chemistrytalk.org
Instagram: / chemtalk
Twitter: / chem_talk
Facebook: / chemtalkorg
#HomeChemistry #ChemistryExperiment #Copper #Reaction #Elements  Наука
Наука



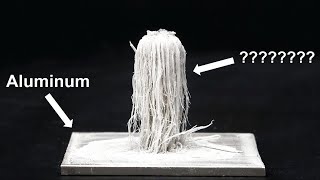





All questions in the comments will be answered. Let us know what your favorite chemistry experiment is, and thank for watching!
I've always had a love for inorganic chemistry and non-biological carbon chemistry. However, gourmet cooking is a great way to apply chemistry and especially organic chemistry. Neat experiment converting aluminum foil to elemental copper.
I did a similar thing with Copper Sulphate and Zinc Oxide. I love displacement reactions! Feels like transmutation.
thanks Aelios, we love them too! We have a few more planned with interesting metals.
I love this reaction it was my favorite early on! I'm re doing this as I need copper powder for stabilizing methyl iodide a bit. Great video!
That's a nice video, thank you!
Thanks for knowledge sharing✌️
I would think with your experience you would know what the NaCl does in the reaction. Count the electrons in the reaction vessel and consider the reactivity of chlorine together with the rectivity series of metals. Copper is the least reactive of all the metals involved, so it is left behind. Sodium is the most reactive, so it creates a side reaction where HCl is created. It is with this side reaction where aluminum and copper trade places in the solution.
Nice one! Thanks for another great experiment video. I did a similar experiment on my channel, but with Copper ii Chloride instead of Sulfate. I recommend that one too!
I will check it out
Heh the more you know about coordination chemistry! I know nothing about it, I’m an organic chemist 😅. I was quite bad at redox when I was in school, but it was always really interesting! Thanks for sharing
Your very welcome!
Nice experiment!thanks lot...sir..pry for good wishes....
thanks Ashok!
Vv nice experiment
THIS sounds interesting
Thank you!
very nice informative video..How is nichrome powder is prepared please can u post any video?
Very interesting for cleaning our environment
Very useful video
Very nice video.i may try that to source my copper for copper acetate.aluminium foil being more abundant at times than actual copper.
I'm curious. if you used Epson salts and electrolysis with a clay pot barrier, and using copper attached (sacrificial) electrodes to manufacture copper sulfate. And then reversed to results as you have done, what would be the net loss of copper?
16:00 i suggest getting actual filter paper (i dunno what its chemically called or wtvr), but i did the same experiment to extract the Aluminium Sulphate, and the filter paper from my lab did the filtration in one go, making the solution clear as water (possibly slightly milky). it goes a long way when conducting experiments for your IB IA's and Extended Essays and just saves you a lot of time!
you most likely used a low retention value (imo
Very nice video, very interesting
thanks Elvis!
Hi, Thanks for explaining the process in great detail and showing the full process till the end. I have one question, Copper Sulphate is highly toxic when poured down the drain so, is the aluminum sulphate generated and all the other waste products safe to be drained down? I will be doing some electrochemistry and basic reactions with it and I am quite worried about copper sulphate waste. Is this method a good way to dispose waste solution of copper sulphate? I would appreciate some opinions and ideas
Your welcome! Aluminum sulfate is the only "waste" and it is very safe to pour down the drain. For copper sulfate solution, you can simply save it for more cool copper chemistry experiments.
Hi there. What's the max temperature the hot plate in the video (four e's model Mi0102003) gets to when you use the thermometer probe?
can u pls tell me, what is the morphology and size of the produced fine copper particle?
I came across your video looking for crystals for grounding a person. What is your use for creating copper? I enjoyed the video 🙂
thanks Shawana!The real question, who would NOT want to make copper?
This is very interesting, does anyone have a proposed mechanism for why the NaCl allows the copper sulfate to bipass the oxide layer?
Great question! The micro-thin oxide layer imparts remarkable resistance to aluminum. The problem with trying to bypass the passivation layer (oxide layer) of aluminum is that it repairs itself very quickly. Yet, chloride is somehow able to do it. Several theories have been proposed why the chloride ion has this effect, but most have not help up to further testing. So even though this has been thoroughly studied, there is no consensus. All that is for sure, is that the chloride ions attack the oxide-layer, penetrate it and somehow reach the oxide-metal interface.
Can i use a liquid copper sulphate solution? Will it work the same?
I think copper sulphate is one of the least problematic chemicals you'll run across. It's often found in child chemistry sets and cat litter. Yes, it's a chemical. There are plenty of chemicals that can harm you. It's why I don't make spaghetti with USB cables.
can we changed into wire form and how? can we alloy it?
Please tell me use of pinch of sodium chloride.
How big size about this copper particle? looks very fine
it creates a fine powder
hi i need ur help i tried to make some cu sulphate by using electorlysis copper in h2so4, bu t i dont realized it was ALUMINUM COPPER COATED WIRE not pure copper and my mistske i used too much ampere@ 12 V from dekstop psu and solution become vigorously reacted till next morning even whrn i unplug cord, what do you think then reaction it was? can i recover my copper as powder?
Aluminum will react vigorously with sulfuric acid to produce aluminum sulfate. You need to react the copper with hydrochloric acid and hydrogen peroxide.
can i use this copper powder for making copper conductive ink, thanks alot.
It's pure copper, so you can use it anywhere you need to use pure copper
Hi. I hope you're well. We did this experiment at my school. We added CuSO4.5H2O to H2O and we also added HCl instead of NaCl. Can you please help me with the balanced chemical equation...Do we also include the water and HCl used and the H2O released?
Can you make an electrical wire from this alloy
Is this copper nitric acid pass ??
wow
Can these be mass produced
we did this at school i had fun
hi JJ, that's great! did they use this video for inspiration, or was a separate idea?
Does the amount of copper metal realized exceed the value of the constituent components used in the experiment?
Actually I think I answered my own question. Bags of copper sulfate on Amazon ar right at the same price per ounce on the market; $0.23 per ounce. There is one bag that is selling for just over $0.20 per ounce. It would take a lot of material to make it worthwhile. If it could be obtained at wholesale prices then I should think that would be quite worthwhile.
Hi, is it possible to torch it to see the metal?... Thnxs
Hello So Mi! Can you explain the question?
Yes. When you torch the copper metal you’ll see a lovely green and blue colour in the flame due to electron excitation :)
Does the heat in the powder makes apear the metal?
Ciao che metallo e prezioso
If I’m doing this at home, can I dispose of the waste in my kitchen trash bag?
hi Katie, good question. Yes any waste from this experiment can be disposed down the drain with plenty of water, or in the trash. We do suggest the people keep the copper powder in a vial or small jar/bottle for future experiments.
Hi, what do you mean by an old salt? ..do you mean a sea salt ?
You can use table salt or sea salt.
Can you use that copper to electroplating
Sure!
Sir have one doubt. (What about that stick bar) i can't understand kindly reply me That name
can you explain?
Is Ryan going to be in any of your videos?
Ryan will be in one soon. Thanks for watching and for the comment! Are you going to try the copper / aluminum experiment?
Sir ,how to make copper sulphide ?
Burn copper powder and sulfur, or react copper sulfate with sodium sulfide
Wouldn't it be great if copper doubled weight when turned back to metel
We wish it would!
This copper rect to nitric acid
do I need a heat plate for this?
no, you do not
Is there a monetary gain?
No, the cost of this is roughly the same as powdered copper off amazon
..Not fully understood, but by you)
اش ذا راسب ذهب
you enjoy thermites
its not the copper penetrating, its the removal of the oxidation layer, you could try baking soda instead of salt, you made an electrolyte from the aluminium and copper sulphate
the reactive part is the aluminium, it takes the initiative, if its exposed to the sulphate, this can be used as a battery chemistry
@@Jkauppa thanks for the great idea
Where is the copper metal?
You can see it at 12:22 at the bottom of the beaker, and at 18:08 in the filter paper
I would like to have seen you melt the copper and carbon powder with an oxy-acetylene torch at 1083 deg celcius.@@ChemTalk
Me too
I'm in NZ we can't even get copper sulfate here. :(
Make some
Off you got car battery drain the fluid then you got watered down sulphuric acid, just need some copper and battery and wire then your on your way to make some.
@@lauraramage261 thanks. I'm looking for this sort of home chemistry, the Makers home chemistry book I bought is absolute garbage.
why?
Ciao che metallo e
Quanto vale
Every chemist I know says they'd never make C10H15N
Atleast they won't publicly admit it. How about you?😉
Copper and aluminum are 2 natural elements. One cannot become the other… all you’re doing is super complicating a simple electrolysis process
Not super complicated.... it's just a single displacement reaction.