Orbitals: Crash Course Chemistry #25
HTML-код
- Опубликовано: 8 фев 2025
- In this episode of Crash Course Chemistry, Hank discusses what molecules actually look like and why, some quantum-mechanical three-dimensional wave functions are explored, he touches on hybridization, and delves into sigma and pi bonds.
Pssst... we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: apple.co/3d4eyZo
Download it here for Android Devices: bit.ly/2SrDulJ
--
Table of Contents
Molecules: Clumpy Globs... 0:18
Quantum-Mechanical Three-Dimensional Wave Functions 3:06
S & P Orbital Hybridization 5:27
Sigma & Pi Bonds 7:32
Hybridized Orbitals 5:52
Crash Course is on Patreon! You can support us directly by signing up at / crashcourse
Want to find Crash Course elsewhere on the internet?
Facebook - / youtubecrashcourse
Twitter - / thecrashcourse
Instagram - / thecrashcourse
CC Kids: / crashcoursekids








Pssst... we made flashcards to help you review the content in this episode! Find them on the free Crash Course App!
Download it here for Apple Devices: apple.co/3d4eyZo
Download it here for Android Devices: bit.ly/2SrDulJ
k
Why crash course in chemistry
It's blasting course 😂😂😂
I appreciate how many times you say "but why does that happen?" "I wanna know why that is the way it is" because its so helpful when im trying to picture what's going on. I can picture everything more clearly now. everything fits together. but its still confusing stuff, especially in my college intro course, but the "why" is so important when trying to understand something
I shall not rest, until I understand the content of this video! May there be lots of coffee!
+Carlos F. Banegas YOU DA REALEST NIGGA
I said that months ago. I think i got it now lol
I said that a 20 minutes ago. I think I got it now. Lol.
Did you finally get to rest or you still going?
n 1 1st energy level 2 electrons n 2 2nd energy level 8 electrons n 3 3rd energy level 18 electrons - n 4 4th energy level 32 electrons. the closer to the nucleus the lower the energy level. n i being the closest to the nucleus However these energy levels have sub levels s, p , d , f and so n1 = s n2 =s , p n3 =s , p , d and n4 = s , p , d , f , and within these sub levels are orbitals s =1 orbital p = 3 orbitals d = 5 orbitals f = 7 orbitals. each one of these orbitals can accommodate 2 electrons in them that have spin in opposite directions.
Most of you are in high school chemistry... well I'm in Integrated Organic/Biochemistry and he is EXTREMELY helpful. Some college professors are horrible teachers!!!
+Maria Martinez With ya on that my professor is awful. A PhD doesn't mean you aren't a dumbass is what my favorite bio professor once told me.
+ALLIED7621 OMG you just made my night!!!! bahahahaha
It's true though some of these professors PhDs are suspect, no way in hell you can barely explain the material to me and you completed the program yourself lol
+Maria Martinez You can be smart, but not everyone can teach. And that goes for them to...
+The GM Well, true, but if they aren't good at teaching, they shouldn't be in the position where that's their job.
I wish these kind of light-hearted and aesthetically pleasing animation videos existed for more advanced topics in chemistry, like molecular symmetry and group theory for example.
what are these words coming out of his face
C.H.E.M.I.S.T.R.Y. Welcome in advance. 😆
try to go for the subtitles
Kunal Yadav he meant it as a joke lmao
😂😂😂😂
I found this part one of the worst in chemistry but it is also one of the most fascinating ones.
About to fail my AP Chem test tomorrow!!
Dan Chen Same, HIGH FIVE!
Anita Dick same here
+Dan Chen bro i already failed most of my ap, stops being a big deal after a while. PS im not actually that bad, i just consider a 3 as a failing grade because the cuny system doesnt accept them!
Same
me too
Guys. He wanted to CHILL US OUT SO HE TALKED ABOUT ICE CLOUDS AND EUROPA'S ICY SURFACE IM BOTH SO IMPRESSED AND SO MAD.
+CFHistory I feel you brah
NOOO NOT TUMBLR IM NOT READY TO BE A BEAUTIFUL BUTTERFLY
oh
CFHistory That was so cool tbh
but are you chill
I've been watching Crash Course since sophomore year of high school. Here I am, years later with a Molecular Biology degree, studying for the MCAT, and I still refer back to your videos for quick, easy (and entertaining) reminders of foundational stuff. Thank you!
"Weird stuff is my favourite stuff cause it means interesting questions". I found my spirit animal.
Lol 😂
That's so me!
Eminem , you have got a huge competition.
You all listened rap God , but here comes CHEM. GOD
lol best comment on this channel
you india you lose
😂😂😂😂😂
😂😂
Put it on 2x and you have CHEMISTRY GODDDD
there we have it guys, water is bent.
NoblexSoul No need for Water benders then. Poor Aang.
WATER IS NOT WET!!!
Omar Abdelkader Who are you talking to
All this talk of water go me bent!
Balloons were such a good way to explain it, that fit in my mind so well
Indian?
I'm in college taking chemistry. My professor is nice, but she sucks at explaining! The damn book does a better job! Still nice person lol. This did not exactly cover what was I looking for, but the information was useful.
+Alex Romero I know right? When a teacher is really nice but she can't explain well and has broken English... I feel bad.
It happens. Being nice in attitude, and being bad @ teaching.
I'm straight outta middle school and just watching this for fun
Alejandra Romero Omg I’m in the same boat! My professor is really nice too but like she doesn’t explain anything well lol I wanna withdraw from the course but my parents and advisor said not to.. I’m so mad.
sameee
put the speed to 0.5 and you'll get drunk Hank explaining science.
Alessandro Manolo g
Thank you for the very needed laughs
why am I so entertained by this? XD
and also try putting the speed to 1.5
Especially at 1.11 it's gonna be awesome
i thought i could get slightly away from my stats class by watching chemistry, but when i saw sigma, i almost died
Enh, they mean two different things though.
yeaaah....exactly where i was going
Sigma hahaha. Too classic.
when you realize that high school chemistry is teaching you nothing
this is better than a months worth of school
Amen
I wish they taught me this in high school so that I could've shut up and learn some simple stoichiometry
They taught me this in like 10th grade
DANDAN THE DANDAN
“Simple stoichiometry”
Sorry, those words don’t work together.
My AP Chem teacher tried to teach this to us in like the last 30 minutes of class. I unfortunately failed to grasp it
ay we got the same profile pic lol
SEBASTION!!!!!!!!!!
Wait, no i should be focusing on electronic configuration, not a hot demon...
My god. I wish I watched this a year ago. This is something that I have been trying to piece together for years now in chemistry class, a visual for /why/ the VSEPR shapes actually occur on an orbital level, what hybridization really is and why it happens, what pi and sigma bonds really look like... And here it is, delivered all in one, cohesive beautiful video. Thank you.
Today learned that a Balloon Artist would not make much of a living selling representations chemical bonds and electron orbits. Not as marketable as swards and dinosaurs.
I love this comment.
fartzinwind L
fartzinwind i love you. Marry me.
I didn't know that swards were in such high demand. I guess people like grass.
I love this comment so much
to the people who are having trouble keeping up..if i was having trouble i would make sure that i understand all these ideas really well before i try to study through these videos.. he is going through them really fast.. if u don't have simple understanding of these ideas u would definitely get lost. but they are gr8 videos to use them as reviews
Anyone else cramming for their mid-terms?
Yep
Tanisha Kore Better question;
Anyone NOT cramming for mid-terms?
Bloopys Kay Yup!
LOL i got finals this week
My teachers have been lazy so I haven't gotten my grades yet (a bit more than a week later)
-looking at me in the eye " or mybe just try to pass a test"
I'm British and studying for my Chemistry AS and I have to say whilst this is a bit beyond my level, it's nice to go above what you need to to get used to stuff
+Jadon Hato We had to do this is in my first year biomedical science degree. I didn't do chemistry A level so I had to go from essentially no knowledge to this in a few weeks.
When you realize you've watched over 20 videos on orbitals and you still don't get and you have a test on it tomorrow
The animation was beautiful and this video was so pleasant to watch. I was stressing a lot about my exam and I'm still stressed but Hank's teaching style makes me smile. Thank you!
Hank, you just managed to make it so much more interesting and relevant than the last 2 years of chemistry have... Thank you!
Some people think Hank speaks too fast. Try watching this at 2x, using RUclips or Chrome's Video Speed Controller extension. I can understand maybe 90% of what he's saying at 2x, but I only understand about 10% of that! Chemistry really is the hardest subject ever! I swear, even calculus is easier than this, and much easier than organic chemistry! Freakin’ hell!
wtf is it weird that i find it easier to understand at 2x speed?
ITS SO INTERESTING THOUGH
i always liked chemistry better
I find it strange that this is taught in introductory chemistry. I think it takes an actual understanding of quantum mechanics for this information to feel relevant.
You haven't done much calculus if you think this level of chem is harder than it
This was simply brilliant! I had to go through it 4 times, with breakouts to userstand some terminology more in depth, then return to this video. Now I am so grateful !
This youtube channel is literally the only way I have passed any of my courses since high school. You guys got me all the way to college and helped me pass my world civilization final yesterday and now I'm hoping for a repeat for my chem final tomorrow. :) You guys are a blessing!!! :')
so ready to show my D in AP Chemistry to the UCs yay!
riveting
I don't think they like seeing the D mate.
I’m in AP Chem rn is the exam bad?
probably
@@linusbantilan7106 i disagree lol
"Weird bags of mostly water" hahahha I see what you did there- nice Star Trek reference! (although I think the line was "UGLY bags of mostly water")
Hank and Crash Course,
You did your absolute best to summarize an entire chapter of college chemistry into ten minutes. Knowing the material already, this was a great refresher. You're fantastic people. Keep it up.
شكرا جزيلا على انكم تقدمون لنا ترجمة باللغة العربية. هذا تقدم رائع جدا لانكم تقدمون هذه المحاضرات لكل الناس بكل اللغات المنتشرة حول العالم 👌 اتمنى لكم المزيد من النجاح والتوفيق
Thank you so much, Hank! The internet is my refuge when I can't understand topics at college.
Now I understand why I didn't understand orbitals in high school. My head would have exploded if my teacher had presented it to us this way.
Thank you for explaining what a node is - which you never did.
When you watch a complicated video at 2x when it is already confusing enough at 1x.
I'm taking Gen Chem 1 in college, and these videos have literally helped me pass my class better than my professor's lectures...... I don't know if thats good or bad, but I ADORE the crash courses!
Despite his 200 word per second speech, I liked how he described us as massive groups bonds and atoms watching youtube and passing exams XD.
Thanks for the ballons demonstration. It made me happy! lol
the sound effect of the electrons bonding is too good! ahahahha
watching this so I don't have to read mu boring chemistry book, thanks Crash Course!
you must read your textbook guy,
the chart at 5:07 single-handedly saved my science career
I wonder how I couldn't discover this gorgeous channel during its journey from 0 to 8 million.
you just did in 10 minutes what my teacher couldnt do in 2 weeks in class over this chapter.
You know, ever since I know that electron is bot wave and particle, I always wonders what it would look like. The example with telephone chord was really helpful in explaining it simply.
Great job, Hank.
For those who are thinking that this was faster than usual, IT WAS!! But, you can watch it over and over again. I guess, Hank had the challenge of packaging all this information into an 11-minute long video, so, this is the result! I am quite confident very few people could replicate or improve this.
THANK YOU, HANK!!!!!!!!!!!!! After years of struggling with this concept (I'm majoring in Chemistry and this is the third year I hve taken Chemistry courses), I think I finally get it. I can't believe I made it to Physical Chemistry and O-Chem 2 without actually understanding this, but now I think I might be able to pass my next exam.
A fellow chemistry major amongst a sea of children lol jk. Same, I'm taking inorganic chemistry and I had to come back to the basics.
Hank green has single handedly saved my school career😍 I actually love this guy
Finally something in Crash Course Chemistry I can understand. 'Cause it's geometry-y.
I had to look this up for some homework and every site I visited seemed to be speaking in tongues. Thankfully, I found this, which has now made it all so much easier. Great job, great channel.
Crash Course Chemistry is really good. This episode was particularly well done. Fascinating.
Hank you're saving me, this corona virus cancelled regular college classes and my chem class was hard enough already. Thank you!
The electrons squeak when the combines,that's cute😂
100 minute lecture in 10 minutes. Real crash course!
My brain after watching roughly 7 times:
🤪🤪🤪 greek wut
I'm majoring in chemistry in university and I'm in my first year. My exam is in a few weeks and these videos are really helping me quickly recap the chapters I just studied. Love it
oml rip my chemistry grade after tomorrow's test
These Crash Course videos are by far the best ones on youtube for understanding. Great production value, and to-the-point.
That was very informative... loved the dsp2 hybrid look, TFS!
I'm in college organic chemistry and I still come to Hank 1st when I'm confused
When i studied these things at school, nobody told me its called quantum physics. Am glad they didn't.
These are so very informative, without being too overwhelming too. I wish education systems could teach as efficiently as this method.
As med student ... This chem course is useful *__*
Wtf this is only useful if you have no experience whatsoever in the field of basic quantum.
+Ed Mar. in my country studyin'this is not part of the curruculum
+Dalip Baitha Sir pls don't comment until u know everything about India.
+Eddie Mar Medical doctors aren't quantum mechanics physicists.
India is such a weird place...I hate to say... but its more bad than good
You have no idea how much this helped me
it’s been 1 yr and I’ve been trying to learn what are orbitals 😭😭thank you smmm
So, you guys ready for that AP test on Monday?
this is my prep lmao
+Corey Lando totally going to fail it but sure! go AP exams!
+Corey Lando I'm literally going over all of these videos the day before -_-
F f f f f f f Noooooooo ahhhhhh
I'm dreading it
Helped me learned this all in a non-messed up way for the first time
Yesterday in chem we were learning about this and now I'm more confused than when my teacher was teaching it.
I watched Kahn academy and they explained it reallllly slowly, it helps on tough subjects to watch multiple different teaching methods. I like this guy cause he goes fast and right on the money, but for extremely complicated information, you need to slow down with a diff guy.
Ni Yao I'm doing this opposite, the khna academy guy was getting off topics and I got lost in space lol
I never took a chemistry class in my life; it's taken me about a month but I finally understand what he us saying in this video and it is awesome!
I understood what 'quantum mechanical probabilistic distribution of electrons in space' meant quite easily. I must be a genius....ok. I'm kidding. I understood it because I read the whole chapter on this in my text book but Hank's always hilarious and I still don't understand Chemistry that much. By the way, De Broglie sucks...ok he doesn't...but he does.
hemangini gurupriya CBSE or state board?
Abhijeet Viswa No we don't. It's really different for both the boards.
Abhijeet Viswa By we, I was talking about my board. Nevertheless, my statement was not accurate and I would like to apologize for it.
Uh...I'm from Andhra and it's Board. Sorry for the late reply man. CBSE here is different, they focus more on the application level of things and usually find theory easy. This was told to me by a friend who studied CBSE syllabus.
hemangini gurupriya Same here. I used to be in CBSE but changed to the state board.
Thanks Hank ! I am no longer confused :)
"Clumpy globs of probable electron locations"...anybody else think of "a big ball of wibbly-wobbly, timey-wimey...stuff"? :)
~:~
(slow clap)
(really slow clap)
(A clap so slow it became fast again)
(slower clap)
(single lone clap)
Gosh, I miss watching these videos in class in middle and high school. Wish I could go back to the good old days.
magic :) with this I might actually get into college :D
I talked about what I learned from this video in an interview for a chemistry degree at a Uni, and they were impressed and gave me a better offer, so thank you crash course chemistry! such a cool video:)
Pause at 8:13 hahahaha-ahem apart from that thank you Crash Course, what would i ever do without you :D
Corvus 😂😂😂😂😂😂
Okay I want to just say that this got me through my Chemistry chapter test so thank you so much Hank! :D
Learning this in grade 9. Gotta understand this good 😓
+ahad Chaudhry AP chem as freshmen, TURNUP
Grade 9 stresssssssssss
BreadcrumbzX Lol dude this should be piece of cake for you then XD
+ahad Chaudhry me too :D
+ahad Chaudhry Not always the case. While you may be learning chemistry that is more advanced for your grade level, these videos do not go very in-depth towards the mathematics behind it (nothing regarding quantum mechanics was discussed, other than a generalized statement regarding wavefunctions). To learn the basic theory behind a subject is much easier than understanding the theory fully.
The animations are so cute, yet very clear in what they're trying to communicate
Yes, I'm just trying to pass a test. (Thanks for the video, I mean it, really~!)
Someone tell my A-levels chemistry teacher the drawing everything on whiteboard does not work at all please~ XD
This is one of the best episodes in the series!
Why does this guy look exactly like the guy who teaches the history crash course
I find him the best....i understand better now and hope I get a good mark on my test.
Thanks Hank!!!
this is not efficient for learning, but best for revision maybe. For me.
totally agree. I am an IT guy, trying to understand why octet rule. This video makes it harder to understand p orbital for me
***** right, I am outsider ! And that's why I need a "crash course". This "course" is not a "crash course". Anyways, I managed to "barely" understand this obital stuff via other easier to understand youtube videos
I watched these videos before my Oxford interview and I ended up getting in.. Tyvm
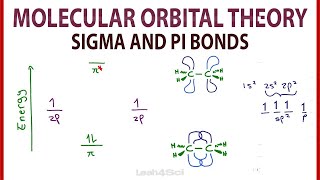
That pi and sigma bond stuff made no sense
sigma is the first bond while pi are the second and third bonds
It also can help if you're told what sigma and pi mean!
The two terms describe the symmetry of the bond that forms. When you get two orbitals meeting end to end like those hybrid orbitals at 7:33 you form a cylinder shape. If you imagine a skewer through the bond as if you were going to spit roast it (through one carbon, through the orbitals and into the other carbon) you can imagine rotating it and it never changes. If you rotate it 20 degrees the orbital remains the same shape, position and phase (black or white) as if you rotate it 100 degrees, etc. This is sigma symmetry. Your pen has sigma symmetry. A drinks bottle has sigma symmetry. A hotdog has sigma symmetry. If you come across this later we call it Cinfinity, i.e. there are an infinite number of positions where you can rotate (C) the object and get an identical looking orbital.
Pi symmetry means that if you put the same skewer through the centre of the two lobes of the Pi bond at 7:48, you would have to rotate the bond 180 degrees to get the orbital to be the same shape again. Anything less than that and the lobes are not in the correct position. There is another element to Pi symmetry that is not represented here. Those p orbitals that make up the Pi bond should have one shaded lobe and one unshaded lobe (representing peaks and troughs of a wave). When you rotate the pi bond with the shaded lobe and unshaded lobe 180 degrees you have phase inversion (i.e. the shaded bit becomes unshaded and vice versa). This is also essential to say something has Pi symmetry. If you look at the yin yang symbol, it also has Pi symmetry. if you imagine putting a pencil through the very centre of it and rotating it 180 degrees, the symbol will be the same except the black bit becomes white and vice versa.
Take this backwards one step, what sort of bond is formed when two s orbitals overlap?
The answer is a sigma bond, because they form a long cylindrical sausage shape, and so it can be rotated to infinity positions and be the same shape.
Pi bond means kissing lobes...
Sigma bonds means... You know...
My lecturer showed us his working using mathematica and showed how orbitals change when we change quantum numbers. So knowing that, I doubt you could understand any behaviour of these orbitals without the math.
organic chemistry please!
Seriously! You want the 3d hybridization of Hydrocarbons and other functionals?
Your videos are soooo much better than the other similar ones on youtube (and a lot less frustrating to watch). "Better" = Contents, graphics, goofy humour, speed, real life associations etc.....Thank you!!!
Orbital V-Sat
the illustrations are amazing
all AP Chemistry has taught me is that i will never do this shit again if i have have the choice
About to bomb my final in CHM1045 at FSU but now, for the first time this semester, I feel like I've finally learned something about chemistry. Thanks CrashCourse.
The hydrogen atoms make that sound. 2:40
This video helped me with orgo 1 better than the textbook and my professor's lectures...thank you!
This is related to the Aufbau principle, right?
yes it is
Yep
I liked the video the second you said " It is going to be awesome "
You are awesome !
Use me as a "im going to fail my ap test this thursday" button
this taught me better than a level chemistry and I'm not even joking
Please, please, please do organic chemistry!
thank you for getting rid of my anxiety for my chem finals, you teach better than my chem teacher