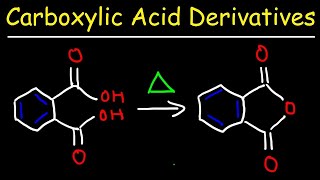
Acid Chloride + Ammonia = Amide (Mechanism)
HTML-код
- Опубликовано: 17 окт 2024
- The nitrogen of ammonia attack the carbonyl carbon (C=O) of the acid chloride. The Cl leaves, as a chloride ion, which goes on to remove one of the H's from what was the ammonia molecule. This leaves -NH2 attached the carbonyl group, which makes the product an amide.
This works for NH3, primary amines and secondary amines. It does NOT work for tertiary amines because they don't have an H to be removed by the Cl.
Check me out: www.chemistnate...








Just finished SCH4U... It was hard, but you made it much easier. Thanks!
Simple yet great and best explanation.
wonderfully simple, great video, thanks so much
Thank You
🤍🤍🤍