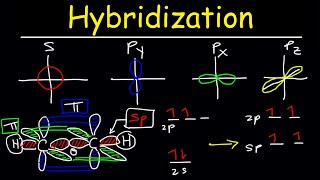
Valence Bond Theory & Hybrid Atomic Orbitals
HTML-код
- Опубликовано: 13 окт 2024
- This organic chemistry video tutorial provides a basic introduction into valence bond theory and hybrid atomic orbitals. It explains how to find the hybridization of carbon atom such as sp, sp2, and sp3. it also explains how to describe the orbitals that overlap to form a covalent bond. In addition, it explains how to determine the number of sigma and pi bonds in a molecule.
Access The Full 33 Minute Video:
/ mathsciencetutor
Direct Link to The Full Video:
bit.ly/3X1ImNI
Organic Chemistry PDF Worksheets:
www.video-tuto...
________________________________________
Join The RUclips Membership Program:
bit.ly/46xaQTR
Full 33 Minute Video on RUclips:
• Valence Bond Theory & ...







Organic Chemistry PDF Worksheets: www.video-tutor.net/orgo-chem.html
Full-Length Exams & PDF Worksheets: www.patreon.com/MathScienceTutor/collections
Access The Full 33 Minute Video: www.patreon.com/MathScienceTutor
Direct Link to The Full Video: bit.ly/3X1ImNI
Bruh this guy works so hard. He learns content to perfection, records edits and uploads almost if not every day on a variety of subjects wich are advanced, not lower study topics. Madlad
@Jordan Vicente can you stop spreading this obvious scam?
@Finley Eric another degenerate bot
Pp
Lol y
This comment 😂❤👀👏💯
I think you deserve some kind of teaching award for summing up a week of lectures in 10 minutes. Thank you for the help
fr
The animations really help me understand this content. I learn more in these 10 minute videos than I do in a 90 minute zoom session.
I took notes on this on HALF of a page while my professor has 20+ slides on it and I still didn't understand it. Thank you!!!
the only thing more impressive than being able to teach all these subjects is the fact that he can actually write on a computer with a mouse
I don't think so. I think He uses a stylus pen. It's IMPOSSIBLE to write with a mouse.
@@beigomaacademymathsclub5873 I draw w a mouse lol
@@SB-qh6cr Yeah, sounds cool. It'd turn out really bad.
He probability uses a wacom tablet
ruclips.net/video/rBSuMooMYrs/видео.html
I’ve spent a couple days struggling on this but all it took was someone drawing the orbitals around the atoms and it clicked immediately. Thank you.
thanks man this will be very helpful for school
we just started learning about this in chemistry last week
Physically cannot wrap my head around this
did you now?
@@Fishylocker lmaooo
You can if you brush up your understanding on Quantum Numbers
I really appreciate these videos so thank you for making them! I am struggling in my college chem courses after being only introduced to very introductory material in highschool. I haven't done very well on my past two midterms, but I am hoping that watching these videos will help me improve my scores!
I have an exam in an hour and a half and u just explained this to me so easily. THANK YOU
Bro is single-handedly carrying me through my calc and chem classes
Who the hell is disliking this helpful video ? 😂
The professors of the students who watch his videos
😂😂
i have never ever understood this concept until now, thank you thank you thank you
For anyone who comes across this comment, you can determine the hybridization of an atom of a molecule by steric number (count the number atoms attached to the central, not directly mentioned in the video). Likewise, if you determine the electronic geometry of a molecule, you can also determine its hybridization. For example, if a molecule has a tetrahedral electron geometry (4 electron domains, steric number 4), then its hybridization is sp3 (1+3=4). Or, an octahedral electron geometry would have a steric number of 6 and have a hybridization of d2sp3 (2+1+3=6). Hope this helps someone.
why do u keep on saving my life
My lectures have never got to make me understand this. The orbitals is when we think of electrons as behaving like a wave!!! 🤯🤯🤯🤯I've never seem to grasp the concept of molecular orbitals until now😭 hoping you can help me pass my orgo chem 2 🙏😍
these videos are carrying through rn! learn more here than actual lectures
He killed it when he say"lets think electrons as a wave"❤
your deadass saving me from failing chem rn thank you so much for these videos
this man is a life saver!
Please i would like to ask from you adding UV and IR spectroscopy
And give us more lessons about biochemistry and thank you for your efforts.
Yes i agree!
He has them. Search his page. ^_^
One of the best tutors I have ever seen btw I got a grade 1 in csec chem thank you very much🥳😘going for a grade 1 in cape now.
Congratulations 🎉
Thank you
This guy must get paid as much a proffessor with how many views he racks in. Homeboy litterally teaches the majority of stem majors at my school.
Omg this is so much easier to understand! When my professor was explaining it I was like this must be the most confusing crap I’ve ever heard! After hearing you explain it, it’s like taking candy from a baby!
You’re the best :)
Does he respond back? It would mean the world to us if he did. Thank you for your hard work, sir, you are the best teacher, out of this world! You deserve to get more subscribers!
is anyone else close to the end of the semester and just feels like dropping out after watching this video to cram for a chem final that's worth 70% of your grade and is 4 hours long?? I hope I'm not alone.
My instructor has a fast pacing. This definitely helps
Very helpful, since it's been 2 years when I initially studied these in my former school. Now in uni, I could not make it through the courses without constantly "learning" old categories again. Funny and in the same time irritating how the human mind is incapable of permanently storing little bits of information. Thank you for these videos, they truly are necessary for me, and I imagine to many others too.
I needed this right now!
Hey there @TheOrganicChemistryTutor, I would like to ask if you have a video tutorial about the basic introduction of valence bond theory and hybrid atomic orbitals? I have looked in your New AP & General Chemistry Video Playlist but I couldn't find it. Please help meee🙏
Thank you so much! It honestly makes so much more sense now. Your soo amazingggggg.
The best teacher thanks
You're a wonderful teacher and your teachings has helped me alot.. thank you so much
Also sir, I had a query. there used to be another video on valance bonds and hybridisation which was about 1hr long, I cannot seem to find that video of yours. that video really helped me alot and I at that moment had not taken proper notes from that video. did you delete that ideo by any chance?
Once again thank you for the wonderful explanation of every topic ☺
thank you for saving my exam grade
To be honest if someone never knew about mathematics and science he/she could just start watching him from the first video and learn everything.
mate you're a life saver
ah i love how the very last seconds of the video just cleared the brain fog haha!
Thank you for your hard work! This video helped to understand this topic
Thank you so much.
You great sir
Thank you very much. This videos really help me so much
You are an awesome motivation
Helpful sir.thaks
Welcome back
Great work 👏👏👏
Thanks science man
If only this came out 1 month ago before my test.....
Watching this few hours to my exam so I don’t read the bulky pdf
Thank you very much. This is really helpful
I tried to find this video for hours right before my exam it turned out that the video got shorten and got uploaded to patreon. Very sad
what’s that
@@drashtipatel6647 a subscription based platform, check his discribtion
am not even american but this helped me a lot thank you sir!
Good video
Learning about this from my curiosity seeing it in the video game Fallout New Vegas. There is a unique piece of headgear called the Atomic-Valence Tri-Radii-Oscillator.
i like your explaination much .
Really helpful
Thanks!
THANKS DO YOU DO VIDEOS FOR INORGANIC CHEM????
Awesome video
Really it's tooooooo helpful for me
Thank you
The deep electron orbit cause the cold fusion.
Good one
Very good
Hey thanks for the video! Can someone explain to me why we consider the electrons in the 2s sublevel to be valence electrons, because I thought that only the ones on the outer most shell (or highest in energy) were the valence electrons. I'm thinking that it's because 2s and 2p are both in the same energy level, so we just count the 2s electrons and 2p electrons giving us 4 total. Can someone confirm if this is the right idea? Thanks!
2s and 2p have the same principal quantum number i.e. their outermost shell is the same...so the outermost shell (not subshell) makes up the valence electrons...which is 4 here
2s and 2p have diff energy levels though...2p has more energy I guess
All excited orbitals have the same energy during hybridization so both 2s and 2p act as valence electrons as they form a sp3 orbital.
you slay every time
Спасибо большое
Hello tutor!
kiinggggg youre the besstt damnn
So the picture on his video I don't understand that specific one . But I'd dont see an explanation of that . Can anyone help ?😊
i like learning chemistry for fun and for the challenge and i have no idea how i stumbled from simple 1st year chemistry to this, and im only in 8th grade lololol
But how to determine which is hybrid and whichs not
can somebody please explain why extent of overlap of orbitals is inversely proportional to size of orbitals ?
Tnx
Idek what I don’t understand but this subject is so confusing
Orange juice and milk 🔥🔥💯
somebody tell me why the most replayed part of this video was the OCT saying "1+2=3"
perfect
3:30 how do you know which electrons are core electrons and which are valence electrons?
Valence electrons are those electrons present in orbitals who's principal qn are equal
The rest are core
Professors should just link his videos instead of wasting their and their students' time...
At minute 5:20 how does he get the 3 p's I didn't understand this part when giving example of methane?
bcs the valence shell (2s and 2p) so you can just count the shape orbital that has been filled by the valence electron only. so if you count, you will get (s, p,p,p)
3:59
🔥
dude how would u mix orange juice with milk?? Gee what is happening out there in the US??
566 Goyette Viaduct
This is a very, very basic and complete introduction. I get you want us to subscribe to your patreon, but this doesn't do Valence Bond Theory justice at all, not even a single mention of Pi bonds
Valence bond theory does not account for delocalization of atoms, so there are no pi bonds in valence bond theory because pi bonds are delocalized
so the no. of sigma bonds = no. of hybrid orbitals
9618 Ullrich Landing
Olaf Crest
616 Hammes Park
Mixing milk and orange juice is crazy
👍
Can you start to teach C C++ Object oriented Python programming?
I love you...
where the fck did the sp3 shit came from
08103 Dee Station
Davis Nancy Anderson Ruth Hernandez Donald
Jones Patricia Johnson John Williams Jeffrey
hızır aleyhisselam diyeeğ anlat hoca anlatt
nah