Molality | Normality | Expressing Concentration of Solutions | grade 10 | exercise 2.9,2.10 solution
HTML-код
- Опубликовано: 17 сен 2024
- 🔬📚 Welcome to our comprehensive guide to understanding the different ways of expressing the concentration of a solution! In this video, we explore the concepts of molality and normality, essential measures that shed light on the concentration of solutions, with a focus on exercises 2.9 and 2.10. Join us as we unravel the complexities of solution concentration and offer illuminating insights into these fundamental principles of chemistry.
🌟 Molality: Exploring the Depths of Solution Concentration
We embark on a captivating journey into the concept of molality, a pivotal measure that defines the concentration of a solution in terms of moles of solute per kilogram of solvent. Through clear explanations and engaging examples, we'll decipher the significance of molality in understanding the behavior of solutes in different solutions.
🌟 Normality: Delving into Solution Equivalents
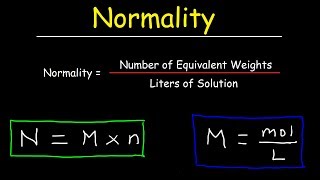
Prepare to dive deep into the realm of normality, a vital measure that expresses the concentration of a solution in terms of the number of chemical equivalents per liter of solution. Unraveling the intricacies of this measure, we'll shed light on its compelling significance in diverse chemical and analytical contexts.
🔍 Exercises 2.9 and 2.10: Practical Application of Solution Concentration
In exercises 2.9 and 2.10, we apply the principles of molality and normality to solve practical problems, offering a hands-on understanding of solution concentration concepts. Through insightful solutions and detailed problem-solving techniques, we aim to empower you with a practical grasp of these fundamental measures.
🎯 Educational Value: Unlocking the Mysteries of Solution Concentration
Whether you're a dedicated chemistry student, a passionate learner of scientific principles, or simply eager to unravel the complexities of solution concentration, this video promises to offer profound insights and practical knowledge to enrich your understanding of these essential chemical concepts.
📢 Engage and Learn Together
If you find the concepts and problems discussed in this video intriguing, don't hesitate to engage with us in the comments section. Share your thoughts, questions, or experiences with solution concentration, and join the discussion to deepen your understanding and inspire others in their learning journey.
🗨️ Interactive Learning: Your Gateway to a Deeper Comprehension
Your active engagement and thoughtful contributions are invaluable in creating an interactive learning space. Let's together explore the intricate world of solution concentration and expand our understanding of these insightful chemical principles.
📚 Practical Insight: Expand Your Chemical Toolbox
By the end of this video, you'll have gained a deeper understanding of molality, normality, and their practical applications, equipped with the essential knowledge to approach solution concentration challenges with confidence.
Join us in unraveling the mysteries of solution concentration and the practical avenues it opens in the captivating world of chemistry.
#molality #normality #chemistryclass10 #chemistry #concentrationofsolutions #molefraction #molarity #SolutionOfExercise2.9 #solutionofexercise2.10 #Grade10Chemistry #chemistry #chemistryclass10 #PPB #ConcentrationOfSolutions
#chemistryeducation #chemistrytutorial #class10 #class10chemistry #solution #concentrationofsolutions #grade10chemistryunit2 #chemistrynotes #chemistrytutorial #chemistrytutorials #ethiopianeducation #chemistryinamharic #ethiopianonlineeducation #bestchemistryandmathstutorial #chemistryforEthiopia #chemistryinamharic #learnchemistry #educationalvideo #education #solutions #concentrations #molarity
---
please don't forget to like, share and subscribe our you tube channel
subscribe : / @bestchemistryandmaths...








Alright you are the best keep up I'm appreciate you
Thank u so much 🙏🙏🙏
You are smart keep it up l am happy
Thank you so much 🙏
Continue my darling . You are the best . But you should be try to improve sound quality
Thank you for your comment, I will try