Comparing E2 E1 Sn2 Sn1 Reactions
HTML-код
- Опубликовано: 16 сен 2010
- Courses on Khan Academy are always 100% free. Start practicing-and saving your progress-now: www.khanacademy.org/science/o...
Comparing E2 E1 Sn2 Sn1 Reactions
More free lessons at: www.khanacademy.org/video?v=12...








I'm finding out this 10 yr/old upload was the greatest early birthday present i could've ever asked for
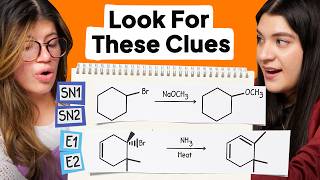
For secondary substrates, E2 usually predominateds, because E2 is
not sterically hindered, while SN2 can be affected by the steric hindrance
When talking about the stereospecificity of cyclopentane , drawing the chair conformation will help you a lot,
With the help of drawing, you can easily determine the stereospecificity of cyclopentan, and then know which one is the major product or the reactant just not react
YES! thank you.
what is steric hindrance?
Actually - both are occurring is not 100% true. Sn2 is occurring less than E2 due to steric hindrance, blocking backside attack (which I'm surprised was not once mentioned in this video and is extremely important for Sn2 reactions). Therefore it's best to say, E2 occurs more than Sn2.
Well isn't this a small world! Hey Eva, it's Michelle! And thanks, I was hoping someone would explain which is the major product.
Woah! That is just awesome. Well I'm glad I helped!!
Eva Gershovich well it is true that both occur so he is right but not in equal amounts is what you are referring to which makes sense. Thanks
Eva Gershovich absolutely correct
Great video. He explained this so much better than my organic professor.
Repetition is the quickest way to memorize something. I used to hate it too then I realized the more he said things the more familiar I became with them.
Thank you so much! You saved my life from my orgo exam :D
I like the black background
THANK YOU SOOOO MUCH!!!! I LOVE YOU!!! Lol..I'm about to keep watching this and your other ones until they sink in!
your videos have helped me soo much this acedemic year. I keep telling my boyfriend that I want to send this person (you) a big fat thank you card! you're awesome and you explain things exactly how they are without complicating things. THANK YOU VERY MUCH. ITS PEOPLE LIKE YOU WHO MAKE THIS A BETTER WORLD FOR EVERYONE :)
Thank you! That really helped a lot! But to determine which reaction is going to take place, wouldn't it also be possible to change the temperature?
I love this man, organic test tomorrow
your videos are so helpful ! thank you
Thanks I'm going to do great on these questions for the test
I love it when I actually understand :)
Very useful content, thanks!
Thank you very much sir. The video was awesome.
Great class! Thanks! Even though you're going all the way up the hill! LOL
you are a very dedicated teacher!
Dane would you mind helping me understand the mechanism for a paper? I'm totally confused
TWO THUMBS UP FOR YOU!! it's a really excellent tutorial video! thank you so much!! keep making videos like these! LOL. God bless you!
CAN YOU PLEASE make a video on what will be considered a strong nucleophile or a strong base? My ochem teacher tried explaining it and it didn't really make any sense.
The way my professor explained it was that typically the strong base or nucleophile will be negative in most cases.
i have my orgo final tomorrow and you cleared up every confusion i had! thank you! btw, how would i know if something is a strong or weak nucleophile?
i was gonna answer this , but then i saw this comment was 12 years ago xd, is organic coming in handing buddy?
I love you! Thank you!
Thank you so much!!!!
"The beta makes it a little dirty." Hah! Love these videos, they are so helpful.
I like the verb nab here. I feel organic chemists should adopt this terminology. ^^
Thank you Sir!
Thank you!
Thanks a lot. 😊😇
thank you...
lol...i didnt realize it was a complete separate video...
Wow. Thank you
What is the criteria to recognize a "strong" base or nucleophile? It seems people just know, which doesn't help me.
its ability to abstract H is stronger, or ability to donate e-. It will be stronger if its partial charge allows for easier split so it gets the e- (i.e Na-OH FOR OH-, or Na-NH2 for NH2- [these anions can now act as nucleophiles and abstract H] ). You can tell if the partial charge is strong by the EN difference between the metal and non-metal
to classify whether a molecule , lets call it molecule A , is a strong base vs strong nucleophile , you have to look at the substituent groups of molecule A . Nucleophilicity parallels basicity except when the said molecule A is sterically hindered , for example , in the case of -OC(CH3)3 , its a very strong base , but its a weak nucleophile because its too sterically hindered to act as a nucleophile , as such , it will ONLY act as a strong base in E2 (mostly) or E1 depending on how stable the carbocation on the target molecule is .
a base is considered a strong base if the pKa of its conjugate acid is 15 or greater.
@@virulentCravings69 This is probably going to sound like a REALLY stupid question, and I know you commented this two years ago... but maybe even if you don't answer, someone else will see it and be able to help. How do we know it's sterically hindered? Maybe that's part of what I'm just not understanding. Does having 3 CH3s just make it big enough to suddenly count as too... complicated? I was under the impression things made bad nucleophiles if they were tiny (like F-) but - OC(CH3)3 doesn't seem like something I'd call small since there's kind of a lot going on there. Is it just because the O- would be similar to an F- (regardless of what's attached) in that H wants to crowd it out? And actually that only applies in a protic solution, doesn't it? So that still means I don't understand well enough in aprotic solution - which is where nucleophalicity matters most OTL
Praaaaaiiiise for this video. 🙏🏼
Wait pls, how come CH3O- is a stronger base than hydroxides??
Nice explained :-) which program do u use?
Nice Explanation
great thanks so much
hey which is faster E1 or E2 and what depends which is faster?
lol, our pot?? ahaha, thanks man! you're awesome! so much sense! :D
GREAT VIDEO
THANK YOU!!!!
major and minor product is jargon that is specifically used for products of elimination reactions (look up "Zaitsev's rules"). Assuming you mean the major and minor product with respect either the SN or E reaction, user Erik D above you has a good explanation.
@jaffey2006 What is a good way to determine the difference between a good base and a good nucleophile? For instance, CH3O- is a good nucleophile but how did you know it was a stronger base?
is it true that the same products are formed between SN1/SN2 (or E1/E2), with the only thing differeng between 1 vs 2 being the rxn mechanism itself?
Yikeees! It's getting real interesting! 😮 😊
Is there other ways or videos that teaches you when to use SN1 SN2 E1 or E2? I found some comments but I think it would be better if Sal makes one. ;D
Does the DMF tert amine remove the hydrogen from the ethanol?
thank you
Doesn't an E2 occur more often than SN2 reactions due to steric hindrance occurring for an SN2 reaction?
thank u.
My notes say that E2 reactions must have a hydrogen that is anti-periplanar. Is there an exception in the case of cyclopentane? All my example drawings are of cyclohexanes or chiral alkanes.
In an Aprotic solvent a strong nucleophile will be a strong base. A weak base would favor an SN1 mechanism.
can any two atoms with pairing valence electrons combine to form a bond??
Is the SN2 the major product since there is no heat in the environment?
Can you be my chemistry professor? This video makes so much more sense than my class on the same topic.
How do you know which is the major product and which is the minor product?
@jordanhop2 DMF is a solvent, it only determines the protic-ness, and the difference between 2- and 1-series reaction
need more details
I thought while both produces may form, the E2 is the major product as an extremely strong base was used and it was a secondary C. However, it requires anti-periplanar geometry with the H, so wrong H extracted I think.
But is there a major product and a minor product..?? :
awesome
It’s worth mentioning that for E2 to occur, the beta Hydrogen should be Anti to the leaving group. ( imagine both of them leaving smoothly in opposite directions) this won’t be the case if they’re on the same side of the ring in which case the reaction will be sterically highly unfavourable.
in what program is he drawing
Ms paint
I love your videos, but I can't really use these as much because you don't involve the pka's. If you do another one you should really involve those for those of us who have professors that want us to learn the reactions strictly through pka's. Keep up the awesome videos though, they are extremely helpful!
The E2 elimination reaction needs to use an anti-periplanar hydrogen, so the wrong hydrogen is being used in this reaction.
which one would be the right hydrogen?
Why is the methoxide ion a strong base and a strong nucleophile?
Is it possible/likely for the CH3O-group to take away the hydrogen atom (proton) attached to the alpha-carbon?
If possible, what would the final result be?
Thank you for making a first time chemistry student with no experience into the top student in class! :)
Do all E2 reactions form shoes?
Aarggg trick question lol. Thank u!!!!!!!
Am I crazy? Given my understanding, this reaction wouldn't create a stereocenter on that alpha carbon. aren't the two carbons in the pentane equivalent? help me
If you count the carbons chained on each side of the alpha, then you'll see that there are different amounts of carbon on each chain, 3 on the right and 2 on the left. Another way to look at it since its CYCLOpentane is if you start counting carbons on each side of the chiral carbon, you will meet on the other side with different amounts of carbins on each side
@@yourface13able That's not true. There are four carbons on either side of the "chiral" carbon. It's not chiral.
does he just know everything and that's how he can do all these videos?
Yes.
"The beta makes it a little dirty" lol.
i wonder how man of the 19k views would have failed their test if not for you
@urdouchbag check out his previous video on the difference: It's called Nucleophilicity vs. Basicity. (Sorry, for some reason RUclips isn't letting me post the link)
The only reaction that would produce a racemic mixture is an Sn1 reaction due to the carbocation that is formed in the transition state.
I Like your pictorial view of SN2, SN1... But they seem very jumbled and disorganized. Still great video
Because both the Sn2 and E2 reactions occur, does that mean that it is a racemic mixture?
i like your handwriting
The major product would be the Elimination reaction right?
Also, how can you differentiate between E1 and E2 when you have the Alpha carbon in secondary instead of primary or tertiary?
+richard kim
For 2° carbons:
SN2 when the pKa of the conjugate acid is less than 11.
E2 when the pKa of the conjugate acid is greater than 11.
SN1/E1 mix if you have a poor nucleophile with polar protic solvent.
+Brittney Ciesa
For 2 degree carbons:
SN1/ E1 mix if poor nucleophile with a protic solvent.
E1 when sterric hindrance by ring is present with above condition
E1 when heat is given or temperature is high.
E1 when nucleophile/ base is bulky
SN2/ E2 mix if strong base/ strong nuleophile and aprotic solvent (but generally SN2 is preferred over E2 if all other factors are same)
E2 when sterric hindrance by ring is present with above condition.
E2 when strong base/nucleophile is bulky
E2 when temperature is high
Thanks Ramesh, very helpful.
Wait, that -OCH3 group is pretty big, how would it do a 180 degree attack on the pentane ring?
Why is methoxide described as "strong nucleophile" for Sn2, and "strong base" for E2?
Why is d methaonic ion a strong nuclophile
Good
i may sound silly...but..what is a concrete difference between an nucleophile and a base....?
bromide has one extra valence electron on its own ; right??
Which is more likely to happen in this example, e2 or sn2 ??
+Sahil Jain Depends on many things, one of them is temperature: if the temperature is higher, E2 would be favored.
+Sahil Jain E2 will be favoured, no need to demand for more information like heat or temperature. There is a lot of sterric hindrance due to the ring and in SN2 reaction, attacking group has to come very close to the carbon atom to donate its electron pair. And, sterric hindrance does not allow the attacking group to come close. So, it simply performs E2.
Nice
"The ß makes it a little dirty," LOL
But really this was very helpful, thank you for the video. :)
Khan academy teaching 360.000 people to draw curved arrows in the wrong way ...
Lol
+Liquoricilicious 360,000. *
I'm not living in the UK/US ... we use a period where I live. :p
+Vito Ferrara that's a comma. What you wrote is 360 people
Khan's drawing it the correct way though, the arrow head faces the hydrogen and the stem comes from the electron?
The oxygen is negatively charged and therefore attracted to the anti-bonding (+) orbital on the alpha carbon. The whole molecule is not doing the attack, just the oxygen atom. It would also need the right trajectory to cause an SN2 reaction.
More examples
12:25 the circled E2 reaction looks like a shoe xD
with a hamster sitting on it!
out of sn2 and e2 which would be the major product?
frigging awesome they are reactions not products...
frigging awesome
Too late to answer..
But it's actually ( mostly )
The zaitseff product would be major ..
But if the base is more bulky then Hoffman would be major ....
Using CH3O- is not really a good example if you want to show an Sn2 because it is such a strong base. An Sn2 would occur, but in such a small quantity, that you wouldn't show it in the mechanism. This favors E2 reaction. The carbon is 2 degrees, the nucleophile is good and strong (not bulky as well) All of these point towards an E2 reaction rather than both. Still love the videos Khan and you're the reason I'm doing well in O-Chem :)
E2 will be dominated in the reaction ..because for sn2 strong nucleophile and weak base like I- is required
+ramanand singh the answer is correct but reason is wrong. E2 will be favored because of sterric hindrance due to the ring.
This is not correct. To decide whether a SN2 or elimination reaction happens we just need more information... We need to know the temperature for example, because there is a strong correlation between elimination and higher temperature... Also solution effects will occur and we need to know the concentration of each reactant.. If the reaction occurs, for example, at a temperature of 150 K, maybe the substitution would be preffered.From this point of view it is quite correct to say, both occure..
If only my professor could be as clear as you, I wouldn't be here
E2 almost always beats SN2 as far as producing the major product when E2 and SN2 occur together. The stronger the base, the more elimination is favored, so the E2 product is the major product by a long way in this case.
But E2 prefers polar protic solvents right??
Farsan Rahman no aprotic
Does cyclopentane contribute to steric hindrance? I assumed because it was a big bulky group, E2 would be favored.